
Introduction
Aldehydes and Ketones of Class 12
Both aldehydes & ketones contain carbonyl group as their functional group.
Structure of carbonyl group
Both aldehydes & ketones have a carbonyl group as the functional group. The carbonyl carbon is sp2 hybridized & it uses sp2 hybrid orbitals to form 3σ bonds, one with oxygen atom & remaining 2 with two other atoms or groups (R or H). All these 3σ bonds lie in same plane at the angle of 120°.
The unhybridized p – orbital of carbonyl carbon form π - bond with oxygen atom by sidewise overlapping with half-filled p – orbital of the oxygen atom.
Since carbon & oxygen has different values of electronegativity, the bond between carbon & oxygen is polar. In fact electron density around the oxygen atom is increased which causes the development of partial positive charge (δ+) on carbon & δ− on oxygen.
Thus carbonyl carbon is electrophilic & carbonyl oxygen is the nucleophilic center.
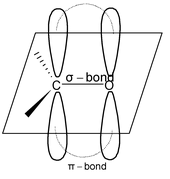
Orbital picture of carbonyl group









