
Isomerism
Carbon And Its Compound of Class 10
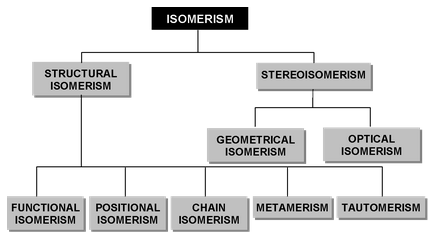
ISOMERISM
Let us consider the structural formulae of the first three members of the alkane series, i.e., the structural formulae of methane, ethane and propane.
If the positions of carbon and hydrogen atoms in these molecules are rearranged, the same structural formulae are obtained. This means that the structural formulae of the first three members of the alkane series remain unchanged, even if the carbon and hydrogen atoms in them are rearranged.
Now consider the fourth member of the alkane series, i.e., butane. In butane, carbon and hydrogen atoms may be arranged differently to give different structures and, hence, different compounds.
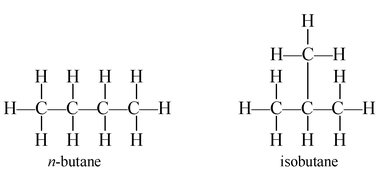
Both n-butane and isobutane have the same molecular formula (C4H10) but their structures are different. In n-butane, the carbon atoms form a longer straight chain, while in isobutene, there is a shorter straight chain and a branch. In the straight chain (n-butane), no carbon atom is bonded to more than two carbon atoms, but in the branched chain (isobutene), one carbon atom is bonded to three other carbon atoms n-butane and isobutene are called isomers.
Organic compounds with the same molecular formula but different structural formulae are known as isomers. This phenomenon is called Isomerism.
CHARACTERISTICS OF ISOMERS:
- All the isomers of a compound have the same molecular formula.
- The isomers of a compound have different structures.
- The physical and chemical properties of all the isomers of a compound differ from one another.
Broadly speaking, isomerism is of two types.
- Structural Isomerism
- Stereoisomerism
(i) Structural isomerism: When the isomerism is simply due to difference in the arrangement of atoms within the molecule without any reference to space, the phenomenon is termed structural isomerism. In other words, while they have same molecular formulas they possess different structural formulas. This type of isomerism which arises from difference in the structure of molecules, includes:
(a) Chain or Nuclear Isomerism;
(b) Positional Isomerism
(c) Functional Isomerism
(d) Metamerism and
(e) Tautomerism
(ii) Stereoisomerism: When isomerism is caused by the different arrangements of atoms or groups in space, the phenomenon is called Stereoisomerism (Greek, Stereos = occupying space). The stereoisomers have the same structural formulas but differ in the spatial arrangement of atoms or groups in the molecule. In other words, stereoisomerism is exhibited by such compounds which have identical molecular structure but different configurations.
Stereoisomerism is of two types:
(a) Geometrical or cis-trans isomerism; and
(b) Optical Isomerism.
Thus various types of isomerism could be summarized as follows.
STRUCTURAL ISOMERISM
Chain or Nuclear Isomerism
This type of isomerism arises from the difference in the structure of carbon chain which forms the nucleus of the molecule. It is, therefore, named as chain,nuclear isomerism or Skeletalisomerism. For example, there are known two butanes which have the same molecular formula (C 4 H 10 ) but differ in the structure of the carbon chains in their molecules.
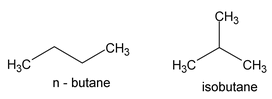
While n-butane has a continuous chain of four carbon atoms, isobutane has a branched chain. These chain isomers have somewhat different physical and chemical properties, n-butane boiling at -0.5 o and isobutane at -10.2 o . This kind of isomerism is also shown by other classes of compounds. Thus n-butyl alcohol and isobutyl alcohol having the same molecular formula C4H9OH are chain isomers.
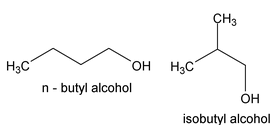
It may be understood clearly that the molecules of chain isomers differ only in respect of the linking of the carbon atoms in the alkanes or in the alkyl radicals present in other compounds.
Positional Isomerism
It is the type of isomerism in which the compounds possessing same molecular formula differ in their properties due to the difference in their properties due to difference in the position of either the functional group or the multiple bond or the branched chain attached to the main carbon chain. For example, n-propyl alcohol and isopropyl alcohol are the positional isomers.
CH 3 –CH 2 –CH 2 –OH CH 3 –CHOH–CH 3
n-propyl alcohol isopropyl alcohol
Butene also has two positional isomers:
CH 2 =CH–CH 2 –CH 3 CH 3 –CH=CH–CH 3
1-butene 2-butene
1-Chlorobutane and 3-Chlorobutane are also the positional isomers:
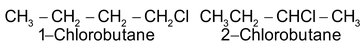
Functional Isomerism
When any two compounds have the same molecular formula but possess different functional groups, they are called functional isomers and the phenomenon is termed functional isomerism. In other words substances with the same molecular formula but belonging to different classes of compounds exhibit functional isomerism. Thus,
(1) Diethyl ether and butyl alcohol both have the molecular formula C 4 H 6 O, but contain different functional groups.
C 2 H 5 –O–C 2 H 5 C 4 H 9 –OH
diethyl ether butyl alcohol
The functional group in diethyl ether is (–O–), while is butyl alcohol it is (–OH).
(2) Acetone and propionaldehyde both with the molecular formula C3H6O are functional isomers.
CH 3 –CO–CH 3 CH 3 –CH 2 –CHO
acetone Propionaldehyde
In acetone the functional group is (–CO–), while in propionaldehyde it is (–CHO).
Metamerism
This type of isomerism is due to the unequal distribution of carbon atoms on either side of the functional group in the molecule of compounds belonging to the same class. For example, methyl propyl ether and diethyl ether both have the same molecular formula.
CH 3 –O–C 3 H 7 C 2 H 5 –O–C 2 H 5
methyl propyl ether diethyl ether
In methyl propyl ether the chain is 1 and 3, while in diethyl ether it is 2 and 2. This isomerism known as Metamerismis shown by members of classes such as ethers, and amines where the central functional group is flanked by two chains. The individual isomers are known as Metamers.
Examples:
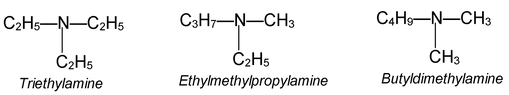
Tautomerism
It is the type of isomerism in which two functional isomers exist together in equilibrium. The two forms existing in equilibrium are called as tautomers. For example, the compound acetoacetic ester has two tautomers – one has a keto group and other has an enol group:
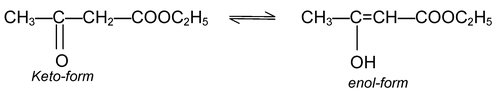
Out of the two tautomeric forms, one is more stable and exists in larger proportion. In above, normally 93% of the keto form (more stable) and only 7% of the enol form (less stable i.e. labile) exist.
STEREOISOMERISM
The isomers which differ only in the orientation of atoms in space are known as stereoisomerism. It’s of two types.
Geometrical isomerism: Isomers which posses the same molecular and structural formula but differ in arrangement of atoms or groups in space around the double bonds, are known as geometrical isomers and the phenomenon is known as geometrical isomerism. Geometrical isomerism are show by the compounds having the structure.
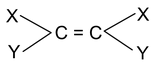
(i) Cis – trans isomerism: When similar groups are on the same side it is cis and if same groups are on the opposite side it is trans isomerism.
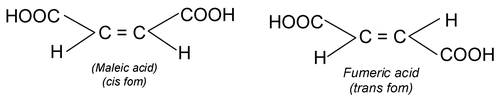
(iii) In cyclic compounds:
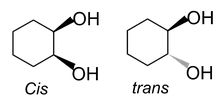
Differentiating properties of cis-trans isomerism
(i) Dipolemoment: Usually dipole moment of cis is larger than the trans-isomer.
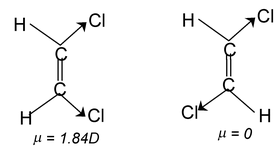
(ii) Melting point: The steric repulsion of the group (same) makes the cis isomer less stable than the trans isomers hence trans form has higher melting point than cis.









