
Some Important Carbon Compound
Carbon And Its Compound of Class 10
ETHANOL OR (ETHYL ALCOHOL):
- Ethanol is the second member of the homologous alcoholic series.
- It is also known as methyl carbinol.
Structural formula:
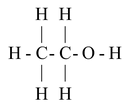
Properties of Ethanol:
Physical Properties:
- Ethanol is a colourless liquid having a pleasant smell.
- Ethanol boils at 351 K.
- It is miscible with water in all proportions.
- It is a non-conductor of electricity (it does not contain ions).
- It is neutral to litmus.
Chemical Properties:
Combustion: Ethanol burns in air with a blue flame to form CO 2 & H 2 O.

Oxidation:
(i) By mild oxidizing agent CrO 3 (Chromic anhydride).
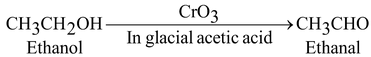
(ii) By strong oxidizing agent (K 2 Cr 2 O 7 + H 2 SO 4 or alkaline KMnO 4 ).
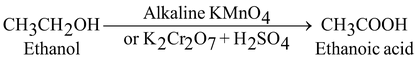
Reaction with Sodium:
Ethanol reacts with sodium to produce hydrogen gas and sodium ethoxide.
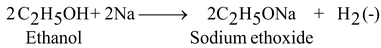
Reaction with carboxylic acids: [ESTERIFICATION]
The process of formation of an ester by the combination of an alcohol with carboxylic acid is known as esterification.
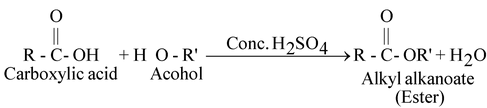
When ethanol reacts with ethanoic acid in presence of concentrated sulphuric acid ethyl ethanoate and water are formed.
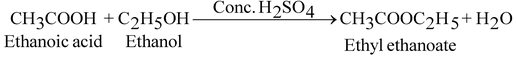
Action with concentrated sulphuric acid:
Ethanol reacts with concentrated sulphuric acid at 443 K to produce ethylene. This reaction is known as acidic dehydration of ethanol because in this reaction, water molecule is removed from ethanol.
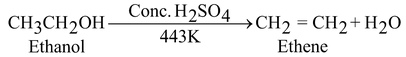
The concentrated sulphuric acid may be regarded as a dehydrating agent because it removes water from ethanol.
SOME IMPORTANT TERMS:
(i) Denatured Alcohol:
To prevent the misuse for drinking purpose, the alcohol supplied for industrial purpose is rendered unfit by mixing it with some poisonous substances like methanol, pyridine, copper sulphate etc. It is known as denatured alcohol.
(ii) Rectified Spirit:
Ethanol containing 5 percent water is known as rectified spirit.
(iii) Absolute Alcohol:
Rectified spirit is heated under reflux over quicklime for about 5 to 6 hours and then allowed to stand for 12 hours. On distillation, pure alcohol (C2H5OH = 100%) is obtained. This is called absolute alcohol.
(iv) Power Alcohol:
Alcohol, which is used for generating power is called power alcohol it consists of a mixture of absolute alcohol and petrol roughly in the ratio 20:80. Since alcohol itself, does not mix with petrol, therefore, a third solvent such as benzene, ether etc, is added as a co-solvent.
USES OF ETHANOL:
(i) Ethanol is a constituent of beverages like beer, wine, whisky and other liquors.
Beer = 3 - 6 % (Ethanol)
Whisky = 50% (Ethanol)
Wine = 10 - 20 % (Ethanol)
(ii) Ethanol is used to sterilize wounds and syringes.
(iii) Antifreeze:
It is a mixture of ethanol and water which has a much lower freezing point than that of water. It is used in radiators of vehicles in cold countries.
(iv) It is used in manufacture of paints, dyes, medicines, soaps and synthetic rubber. Solutions of ethanol prepared in pharmaceutical industry are known as tinctures.
HARMFUL EFFECTS OF DRINKING ALCOHOL:
- If ethanol is mixed with CH 3 OH and consumed, it may cause serious poisoning and loss of eyesight.
- It causes addiction (habit forming) and mixes with blood. It damages liver if taken regularly.
- Higher amount of consumption of ethanol leads to loss of body control & consciousness. It may even cause death.
Alcohol as a fuel:
Sugarcane plants are the most efficient convertors of sunlight energy into chemical energy. The cheap source of alcohol is molasses. It is a dark coloured thick syrupy liquid left after the crystallization of sugar from sugarcane juice. It still contains about 40% of sugar which cannot be obtained by crystallization.
Fermentation of molasses in presence of yeast (which contains the enzymes invertase and zymase) gives alcohol (ethanol).
Fermentation may be defined as the slow decomposition of big organic molecules into simpler molecules in presence of enzymes.
Since alcohol is a cleaner fuel which gives only carbon dioxide and water as by products on burning in excess of air or oxygen, therefore, some countries now use alcohol as a fuel in internal combustion engines in form of power alcohol. Power alcohol is a mixture of absolute alcohol (100% alcohol) and petrol in the ratio 20 : 80. Since alcohol does not mix with petrol, therefore, a third solvent, i.e., benzene is used to dissolve them. It is also used as a fuel in stoves and spirit lamps.
|
|
Drunken driving: Every year thousands of people die and many more are injured as a result of drunken driving. In many countries, police use a device called breath analyzer to test drivers suspected of being intoxicated. The driver is made to exhale and the exhaled breath is passed into the breath analyzer where it is treated with an acidified solution of K2Cr2O7. If the breath contains alcohol, the colour of the solution changes from orange yellow to green. The following reaction occurs :
|
ETHANOIC ACID (OR ACTIC ACID):
(i) Molecular Formula: CH 3 COOH
( ii) Structural Formula:
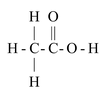
(iii) The IUPAC name of acetic acid is ethanoic acid.
(iv) Occurrence:
Ethanoic acid is known as vinegar, from ancient times. Vinegar is essentially a dilute solution of ethanoic acid in water. The acid is also present some fruit juice. In the combined form, it is also present in many perfumed oils. Ethanoic acid was first prepared in the pure state by Stahl in 1720.
Physical Properties:
- Ethanoic acid is a colourless viscous liquid but has a pungent and irritating smell of vinegar.
- Its boiling point is 391 K.
- It dissolves in water, alcohol and ether. Its dissolution in water takes place with the evolution of heat and decrease in volume of the solution.
- The melting point of ethanoic acid is 290 K and hence it often if freezes during winter in cold climates. Therefore, it is named as glacial acetic acid.
Chemical Properties:
(i) Acidic Character:
Ethanoic acid is a monobasic acid. It has a replaceable hydrogen atom in its – COOH group. Therefore, it neutralizes alkalis.
(A) It reacts with a solution of sodium hydroxide to form sodium propanoate and water.
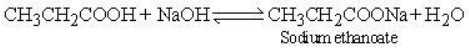
Sodium propanoate is an ionic compound which dissolves in polar solvents such as water, but does not dissolves in non polar solvents such as alcohol, propanone etc.
The aqueous solution of sodium ethanoate is alkaline due to hydrolysis.
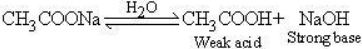
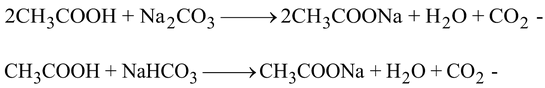
(B) It reacts with sodium carbonate and sodium bicarbonate with the evolution of CO2 gas.
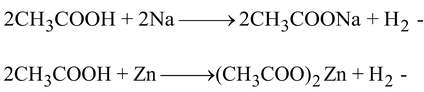
(C) It reacts with metals like sodium, zinc and magnesium to liberate hydrogen gas.
(ii) Ester formation:
When ethanoic acid is heated with ethanol in presence of small quantity of conc. H2SO4 ethyl ethanoate, a sweet smelling ester, is formed.
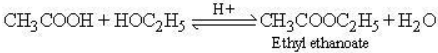
This process of ester formation is called esterification.
(iii) Decarboxylation:
When sodium ethanoate is heated with soda lime, methane is formed.
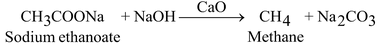
USES:
- Dilute aqueous solution (5-8%) of ethanoic acid is called vinegar, which is used to preserve food (sausage, pickles, etc.).
- Pure ethanoic acid is used as a solvent and chemical reagent.
- As cellulose ethanoate, it is used in making photographic films and rayon.
- Ethanoic acid also finds application in the preparation of propanone, chloroethanoic acid, ethanoates of metals etc.
- It is widely used in the manufacture of textiles.
- It is used in the preparation of white lead.
TESTS FOR ETHANOIC ACID:
(i) Litmus test:
Add small amount of blue litmus solution to the given compound. If the blue litmus solution turns red, it indicates that the organic compound is ethanoic acid.
(ii) Sodium bicarbonate test:
Take a small portion of the organic compound in a test tube and add a pinch of solid sodium bicarbonate. Evolution of carbon dioxide with brisk effervescence shows the presence of carboxylic acid.
(iii) Ester formation:
When a mixture of ethanoic acid and ethanol is heated in the presence of concentrated sulphuric acid, a fruity smelling ester, ethyl ethanoate, is produced.











