
Chemical Reaction
Chemical Reaction And Equation of Class 10
CHEMICAL REACTION
The process involving a chemical change is called a chemical reaction.
or
A chemical reaction is a process which transforms one or more substances into new substances.
or
The process in which a substance or substances undergo change, to produce new substances with new properties, is known as chemical reaction.
Reactants: The substances which take part in a chemical reaction are called reactants.
Products: The new substances formed as a result of the chemical reaction are called products.
For example
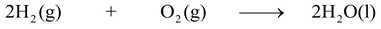
(Reactant) (Reactant) (Product)
In the above chemical reaction hydrogen and oxygen which are written on the left hand side are reactants and water which is written on the right hand side is a product.
A chemical equation not only provides a qualitative description but also quantitative description of the chemical reaction.
For example: Consider the following chemical equation.
2CO + O 2 -→ 2CO 2
The following information is provided by the chemical equation.
- 2 molecules of carbon monoxide will combine with 1 molecule of oxygen to form 2 molecules of carbon dioxide.
- 2 moles of carbon monoxide will combine with 1 mole of oxygen to obtain 2 moles of carbon dioxide.
- 2 × 28 g of carbon monoxide will combine with 32 g of oxygen to form 2 × 44 = 88 g of carbon dioxide.
- 2 volumes of carbon monoxide will combine with 1 volume of oxygen to form 2 volumes of carbon dioxide (only for gaseous-reactants and products) at same temperature and pressure.
Thus, it is possible to calculate the quantities of substances that react and the quantities of products that are formed from a balanced chemical equation. The branch of chemistry that deals with such calculations is Stoichiometry. Thus,
The branch of chemistry that deals with calculation based upon the information provided by mass-mass, mass-volume and volume-volume relationships in a balanced chemical equation is known as stoichiometry and the equation used for this purpose is knownas stoichiometric equation. The word is derived from the Greek word stoikheion (“element”) and metria (“measure,” from metron).
Calculations based on chemical equations
A balanced chemical equation provides the following information regarding the reactants and products,
- Molecular relation between reactants and products.
- Molar relation between reactants and products.
- Weight relation between reactant and products.
- Volume relation between gaseous reactants and products.
Stoichiometric calculations are mainly of three types:
- Calculations based on weight-weight relations
- Calculations based on weight-volume relations
- Calculations based on volume-volume relation










