
Hess's Law
Thermodynamics of Class 11
This law states that the amount of heat evolved or absorbed in a process, including a chemical change is the same whether the process takes place in one or several steps.
Suppose in a process the system changes from state A to state B in one step and the heat exchanged in this change is q. Now suppose the system changes from state A to state B in three steps involving a change from A to C, C to D and finally from D to B. If q 1 , q 2 and q 3 are the heats exchanged in the first, second and third step, respectively then according to Hess’s law
q 1 + q 2 + q 3 = q
Hess’s law is simply a corollary of the first law of thermodynamics. It implies that enthalpy change of a reaction depends on the initial and final state and is independent of the manner by which the change is brought about.
APPLICATION OF HESS’S LAW
1. Calculation of enthalpies of formation
There are large number of compounds such as C6H6, CO, C2H6 etc whose direct synthesis from their constituent element is not possible. Their ΔH0f values can be determined indirectly by Hess’s law. e.g. let us consider Hess’s law cycle for CO2 (g) to calculate the ΔH0f of CO(g) which can not determined otherwise.
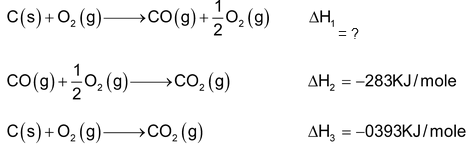
According to Hess’s law,
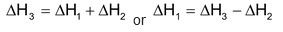
= −393 − (−283) ⇒ −110 KJ/mole
2. Calculation of standard Enthalpies of reactions
From the knowledge of the standard enthalpies of formation of reactants and products the standard enthalpy of reaction can be calculated using Hess’s law.
According to Hess’s law
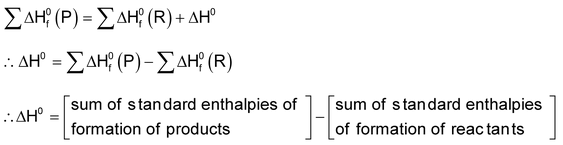
3. In the calculation of bond energies
BOND ENERGY
Bond energy for any particular type of bond in a compound may be defined as the average amount of energy required to dissociate one mole, viz Avogadro’s number of bonds of that type present in the compound. Bond energy is also called the enthalpy of formation of the bond.
Calculation:
For diatomic molecules like H 2 , O 2 , N 2 , HCl, HF etc, the bond energies are equal to their dissociation energies. For polyatomic molecules, the bond energy of a particular bond is found from the values of the enthalpies of formation. Similarly the bond energies of heteronuclear diatomic molecules like HCl, HF etc can be obtained directly from experiments or may be calculated from the bond energies of homonuclear diatomic molecules.




