Variation Of Heat Of Reaction With Temperature
Thermodynamics of Class 11
The heat of reaction depends on the temperature. The relation between the two is known as Kirchoff’s equation.
-
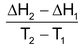 = ΔCP
= ΔCP -
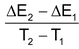 = ΔCV
= ΔCV
ΔCP = molar heat capacity of products – molar heat capacity of reactants (at constant pressure)
ΔCv = molar heat capacity of products – molar heat capacity of reactants (at constant volume)
- Introduction
- Some Basic Terms
- Isochoric Process
- Internal Energy, U
- Mathematical Expression Of First Law
- Enthalpy Of A System
- Second Law Of Thermodynamics
- Gibbs Free Energy
- Relationship Between Free Energy And Equilibrium Constant
- Third Law Of Thermodynamics
- Thermochemistry
- Hess's Law
- Lattice Energy Of An Ionic Crystal (Born–Haber Cycle)
- Bomb Calorimeter
- Heat Capacity And Specific Heat
- Variation Of Heat Of Reaction With Temperature
- Exercise 1
- Exercise 2









