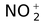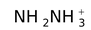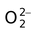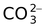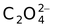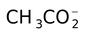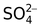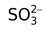IUPAC Nomenclature Of CO−Ordination Compounds
Coordination Compound of Class 12
In general, a complex ion is represented as
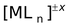
where M is the metal ion, L represents ligands, n is the coordination number of metal ion and x is the net charge on the complex.
There are basically four types of complexes. These are
(i) cation as complex ion. For e.g. [Cr(NH 3 ) 6 ]Cl 3
(ii) Anion as complex ion. For e.g. K 3 [Fe(CN) 6 ]
(iii) Cation and anion both as complex ion. For e.g. [Pt(Py) 4 ] [PtCl 4 ]
(iv) Neutral complexes. For e.g. [Ni(CO) 4 ]
The complex may be of any type (mentioned above), the method of systematic naming of coordination compounds by IUPAC follows the same set of rules.
(a) Cation is named first followed by the name of anion.
(b) There has to be a gap between the cation's name and anion's name. There should be no gap anywhere else i.e. the complete name of co−ordination sphere is written as one word without spacing and punctuation.
(c) Only the first letter of the name of complex should be capital. Rest all letters has to be small.
(d) The number of cations or anions in a complex are not mentioned.
(e) Naming of complex species: Ligands are named first in the alphabetical order (regardless of their charge) and then the central metal atom or ion followed by the oxidation state of the metal in parenthesis in Roman numeral (except for zero). Zero is written in Arabic numeral (0).
(f) If a ligand is repeated more than once, then prefixes di, tri, tetra, penta, hexa etc. are used. For example, −[−(NH 3 ) 5 ] is written as pentaammine. Care must be taken to write 'a' twice (one for penta and one for ammine) and ammine with double 'm' (in order to distinguish it from organic amines).
(g) In deciding the alphabetical order for ligands, prefixes di, tri, tetra etc. are not considered. Alphabetical order is decided by the first letter of the ligand's name.
(h) In case of ligands having di, tri, tetra in their names (inbuilt, which actually means 2, 3, 4) or organic ligands, if they are repeated more than once, prefixes used are bis (for two), tris(for three), tetrakis (for four), pentakis (for five) etc. After writing bis or tris, the name of the ligand is to be written in parenthesis. For example, (en)2 is written as bis(ethylenediamine).
(i) Negative ligands end with 'o' and positive ligands with 'ium'. There is no special ending for neutral ligands.
(j) Naming anion complexes: For naming anion complex, suffix 'ate' is put after the name of metal atom i.e. written as metalate. For example, platinate, ferrate, nickelate, cobaltate, cuprate, wolfrate, argentate, aurate, iridate, chromate, osmate etc. Remember that for those metals whose latin names are available (except for mercury), the suffix 'ate' is used after that while for those whose latin names are not available, the suffix is used after normal metal names. No such suffix is used for metal in cation complexes and neutral complexes.
(k) For naming a complex ion, ion has to be written after the name of that complex ion.
(l) If any lattice components such as water or solvent of crystallization are present, these follow the name and are preceded by the number of these groups in Arabic numerals, separated from the name of these groups by a hyphen.
(m) For bridging complexes: Bridging complexes are the one which have bridging ligands and bridging ligands are the one which joins two metal ions. For naming bridging ligands, µ is placed before the name of ligand in order to distinguish it from the normal ligand. If there are two or more bridging ligands of the same kind, this is indicated by di−µ−, tri−µ−etc. If more than one kind of bridging ligands are present, then they are listed in the alphabetical order. If a bridging group bridges more than two metal atoms it is shown as µ3, µ4, µ5 or µ6 to indicate how many atoms it is bonded to.
(n) Ambidentate ligands: There are some ligands which have more than one donor atom and such ligands can coordinate with the metal atom by any donor atom. For example, −ONO is called nitrito and −NO2 is called nitro,−SCN is called thiocyanato and −NCS is called isothiocyanato. These may also be named systematically as thiocyanato −S and thiocyanato−N, (to indicate which atom is bonded to the metal).
(o) When writing the formula of complexes, the complex ion should be enclosed by square brackets. The metal is named first, then the coordinated groups are listed, in the order−negative, neutral and positive ligands (alphabetically arranged according to the first symbol within each group).
(p) Naming of complex with metal to metal bridging
For such complexes, prefix bi− is placed before the name of the metal atom or ion.
For example,
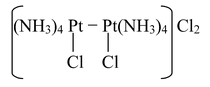
Octaamminedichlorobi−platinum(II) chloride
Note:
The stable oxidation states of some of the transition metals of the three series are given below. These would be helpful to find the oxidation states of the metal ions while naming complexes having cation and anion both as complex species.
(i) First transition series
|
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
|
+3 |
+2, +3, +4 |
+2, +3, + 4, +5 |
+2, +3, +6 |
+2, +3, +4, +7 |
+2, +3 |
+2, +3 |
+2,+3 |
+1, +2 |
+2 |
(ii) Second transition series
|
Y |
Zr |
Nb |
Mo |
Tc |
Ru |
Rh |
Pd |
Ag |
Cd |
|
+3 |
+4 |
+3,+5 |
+6 |
+4, +6, +7 |
+3 |
+3 |
+2,+4 |
+1 |
+2 |
(iii) Third transition series
|
La |
Hf |
Ta |
W |
Re |
Os |
Ir |
Pt |
Au |
Hg |
|
+3 |
+4 |
+5 |
+6 |
+4, +6, +7 |
+3, +4, +6 |
+1, +3, +4 |
+2,+4 |
+1, +3 |
+1, +2 |
Names of Positive ligands:
|
Ligand |
Name |
|
|
nitrosonium |
|
|
nitronium |
|
|
hydrazinium |
Names of Neutral ligands:
|
ligand |
Name |
Abbreviation |
|
H 2 O |
aqua/aquo |
− |
|
NH 3 |
ammine |
− |
|
CO |
carbonyl |
− |
|
NO |
nitrosyl |
− |
|
CS |
thiocarbonyl |
− |
|
NS |
thionitrosyl |
− |
|
C 5 H 5 N |
pyridine |
(py) |
|
NH 2 (CH 2 ) 2 NH 2 |
ethylenediamine |
(en) |
|
CH 3 NH 2 |
methylamine |
− |
|
C 6 H 6 |
benzene |
− |
|
N 2 |
dinitrogen |
− |
|
O 2 |
dioxygen |
− |
|
Ph 3 P |
triphenylphosphine |
− |
|
CH 3 COCH 3 |
acetone |
− |
Names of negative ligands:
|
Ligand |
Name |
|
H− |
hydrido |
|
O 2- |
oxo |
|
|
peroxo |
|
O2H− |
perhydroxo |
|
OH− |
hydroxo |
|
F− |
fluoro |
|
Cl− |
chloro |
|
Br− |
bromo |
|
I− |
iodo |
|
|
carbonato |
|
|
oxalato |
|
|
acetato |
|
CN− |
cyano |
|
|
sulphato |
|
|
sulphito |
|
S 2- |
sulphido or thio |
|
HSO 3 - |
hydrogensulphito |
|
S 2 O 3 2- |
thiosulphato |
|
HS− |
mercapto |
|
NH - 2 |
amido |
|
NH 2- |
imido |
|
No - 3
|
nitrato |
|
ONO− |
nitrito |
|
NO - 2 |
nitro |
|
N 3- |
nitrido |
|
N - 3 |
azido |
|
CNO− |
cyanato |
|
OCN− |
isocyanato |
|
SCN− |
thiocyanato |
|
NCS− |
isothiocyanato |
|
HCO - 3
|
hydrogencarbonato |
|
S 4 O 6 2- |
tetrathionato |
|
EDTA (−O 2 CCH 2 )2NCH 2 CH 2 N (CH 2 CO 2 −) 2 |
ethylenediaminetetraaceticacid (hexadentate) |
|
NH 2 CH 2 CO - 2 |
glycinato |
|
CH 3 COCH 2 COCH 2 - |
acetylacetonato (acac) |
|
C 5 H 5 - |
cyclopentadienyl |
The above rules are illustrated by the following examples.
|
[CrCl 2 (H2O) 4 ]Cl |
Tetraquodichlorochromium(III) chloride |
|
[CoCl(NH 3 ) 5 ] 2+ |
Pentaamminechlorocobalt(III) ion |
|
Mg3[Fe(CN) 5 CO] 2 |
Magnesium carbonylpentacyanoferrate(II) |
|
[CoSO 4 (NH 3 ) 4 ]NO 3 |
Tetraamminesulphatocobalt(III) nitrate |
|
[Ag(NH 3 ) 2 ] 2 (SO 4 ) 3 |
Diamminesilver(I) sulphate |
|
Li[AlH4] |
Lithium tetrahydridoaluminate(III) |
|
[Zn(NCS) 4 ] 2- |
Tetraisothiocyanatozincate(II) ion |
|
[CoCl(CN)NO 2 (NH 3 ) 3 ] |
Triamminechlorocyanonitrocobalt(III) |
|
K 4 [Fe(CN) 6 ] |
Potassium hexacyanoferrate(II) |
|
K 3[ Al(C 2 O 4 ) 3 ] |
Potassium trioxalatoaluminate(III) |
|
[Cd(SCN) 4 ] 2- |
Tetrathiocyanatocadmate(II) ion |
|
K 3 [Fe(CN) 5 NO] |
Potassium pentacyanonitrosylferrate(II) |
|
[Fe(C 5 H 5 ) 2 ] |
Bis(cyclopentadienyl)iron(II) |
|
Na 3 [Ag(S 2 O 3 ) 2 ] |
Sodium bis(thiosulphato)argentate(I) |
|
[Cr(C 6 H 6 ) 2 ] |
Bis(benzene)chromium(0) |
|
[CuCl 2 (CH 3 NH 2 ) 2 ] |
Dichlorobis(methylamine)copper(II) |
|
[(NH 3 ) 5 Co−NH 2 −Co(NH 3 ) 5 )] (NO 3 ) 5 |
µ−amidobis[pentaamminecobalt(III)] nitrate |
|
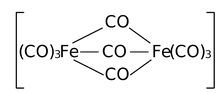
|
Tri−µ−carbonylbis[tricarbonyliron(0)] (di iron enneacarbonyl) |
|
AlK(SO 4 ) 2 .12H 2 O |
Aluminium potassium sulphate 12−water |