
Inductive Effect
GOC of Class 11
Inductive Effect
First let us look at the C − C bond in ethane. This C − C bond has no polarity because it connects two similar atoms. However, the C − C bond in ethyl chloride is polarized by the presence of the electronegative chlorine atom. This polarization is actually the sum of three effects. In the first of these, the C 1 atom have been deprived of some of its electron density by the higher electronegativity of Cl. Secondly, the electron deficiency of C1 is partially compensated by drawing the C − C electrons closer to itself, resulting in polarization of this bond and a slight positive charge on the C 2 atom. Thirdly, the polarization of the C − C bond causes a (slight) polarization of the three methyl C − H bonds. The effect of C 1 on C 2 is less than the effect of Cl on C1 atom.
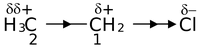
In addition to such inductive effect operating through the σ−bonds in a compound, an analogous effect can also operate either through the space surrounding the molecule or in solution via the molecules of solvent that surround it which is called field effect. A point of distinction between the two effects is that the inductive effect depends only the nature of bonds while the field effect depends on the geometry of the molecule. However, in many cases, it is not possible to distinguish inductive effect with this field effect. But in general, reference to an inductive effect is assumed to include any such field effect.
In ethyl chloride, Cl is said to have an electron withdrawing –I effect. There are other type of groups which exert electron releasing +I effect. Thus, the substituents or functional groups can be classified as electron-withdrawing (–I) and electron-donating (+I) groups relative to hydrogen.
The following is the order of decreasing inductive effects of most common groups.
+I groups :O - , CO 2 - , CR 3 , CHR 2 , CH 2 R, CH 3 , D
– I groups :
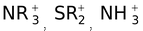 , NO
2
, SO
2
R, CN, CO
2
H, F, Cl, Br, I, OAr, COOR, OR, COR, SH, OH, C ≡ CR, Ar, CH = CR
2
, NO
2
, SO
2
R, CN, CO
2
H, F, Cl, Br, I, OAr, COOR, OR, COR, SH, OH, C ≡ CR, Ar, CH = CR
2
For measurements of relative inductive effects, hydrogen is chosen as reference in the molecule CR 3 – H. If the H atom in this molecule is replaced by X (an atom or group of atoms), the electron density in the CR 3 part of the molecule is less than in CR 3 – H, then X is said to have a –I effect (electron withdrawing or electron attracting). If the electron density in the CR 3 part is greater than in CR 3 – H, then X is said to have a +I effect (electron releasing or electron repelling).
All Alkyl groups exhibit +I effect when they are attached to an unsaturated or trivalent carbon (or other atom). Deuterium is also electron donating with respect to hydrogen. Keeping other things equal, atoms with sp bonding generally have a greater electron withdrawing power than those with sp 2 bonding, which in turn have more electron-withdrawing power than with sp 3 bonding. This accounts for the fact that aryl, vinylic and alkynyl groups exhibit –I effect.




