
COLLOIDAL SOLUTION
Is matter around us pure of Class 9
COLLOIDAL SOLUTION
Solutions in which the size of the particles lie in between those of true solutions and suspensions are called colloidal solutions or simply colloids.
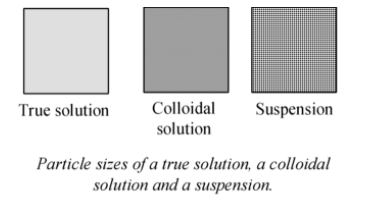
Though colloids appear to be homogeneous to us, but actually they are found to be heterogeneous. So, colloid is not a true solution, therefore, to distinguish it from the true solution, the term sol is used in place of solution (i.e. colloidal sol).
Examples of Colloidal Solutions:
Few examples of colloidal solutions are as follows:
➢ blood ➢ Milk ➢ Writing ink ➢ Jelly
➢ Starch solution ➢ Gum solution ➢ Tooth paste ➢ Soap solution
➢ Liquid detergents ➢ Mist and fog
DISPERSED PHASE AND DISPERSION MEDIUM:
Components of a colloidal solution are the dispersed phase and the dispersion medium.
The solute like component which has been dispersed or distributed throughout a solvent-like medium is called the dispersed phase or the discontinuous phase.
The solvent like medium in which the dispersed phase has been distributed or dispersed is called the dispersion medium or the continuous phase.
|
TYPES OF COLLOIDS:
Colloids are classified according to the state (solid, liquid or gas) of the dispersion medium and the dispersed phase. A few examples are given in table as follows:
Common examples of colloids:
|
Dispersed phase |
Dispersion Medium |
Type |
Example |
|
Liquid Solid Gas Liquid Solid Gas Liquid Solid |
Gas Gas Liquid Liquid Liquid Solid Solid Solid |
Aerosol Aerosol Foam Emulsion Sol Foam Gel Solid Sol |
Fog, clouds, mist Smoke, automobile exhaust Shaving cream Milk, face cream Milk of magnesia, mud Foam, rubber, sponge, pumice Jelly, cheese, butter Coloured gemstone, milky glass |
PROPERTIES OF A COLLOID:
- Heterogeneous Nature: A colloid is a heterogeneous mixture.
-
Size of particles:
Size (diameter) of particles in a colloid lies in the range 1–100 nm
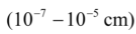 i.e. in between those of true solutions and suspensions.
i.e. in between those of true solutions and suspensions.
- Stability: Colloidal sols are quite stable i.e., colloidal particles do not settle when left undisturbed.
- Visibility: Colloidal particles are not visible to the naked eye.
- Filterability: Colloidal particles cannot be separated from the dispersion medium by filtration. However, a special technique of separation known as centrifugation can be used to separate the colloidal particles.
- Tyndall effect : The colloidal particles are big enough to scatter light passing through it. As a result, the path of light becomes visible. This scattering of beam of light by colloidal particles is called the ‘Tyndall effect’. Lets understand -
When the beam of light from a torch is passed through a true solution of copper sulphate, Tyndall effect is not observed, i.e., the path of light is not visible (Figure a) However, when the same light is passed through a mixture of water and milk, Tyndall effect is observed and the path of light becomes visible (Figure b). The reason for this observation is that the particles of a true solution are so small that they do not scatter light and hence the path of light is not visible, i.e., Tyndall effect is not observed. In contrast, the particles of a colloidal solution are big enough to scatter light and hence path of light becomes visible, i.e., the Tyndall effect is observed.
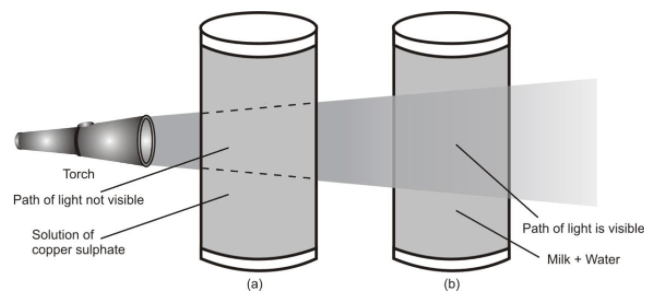
- (a) Solution of copper sulphate does not show Tyndall effect
- (b) Mixture of water and milk shows Tyndall effect.




