

In boiling point elevation, the boiling point of a solvent increases when a solute is added. If a non-volatile solute is added to a solvent, the resulting solution has a higher boiling point than the solvent alone. For instance, the boiling point of a sodium chloride (salt) and water solution is greater than that of pure water. This effect, known as elevation of boiling point, occurs because of colligative properties; in other words, it depends on the amount of solute included in the solvent, not its identity—the more solutes in the mixture, the bigger the increase in the boiling temperature.
Boiling Point Elevation
When a solution is applied, the vapor pressure of the solvent decreases because some of the molecules on the liquid's surface get displaced by solute molecules. This leads to fewer solvent molecules for evaporation, resulting in lower vapor pressure. To achieve equilibrium with ambient pressure, it requires a higher temperature and causes an increase in boiling point. The figure above demonstrates this effect through the example of water and 10-molal sucrose solution. While pure water boils at 100 degrees Celsius under 1atm pressure, the sucrose solution boils at around 105 degrees Celsius. [caption id="attachment_16734" align="alignnone" width="300"]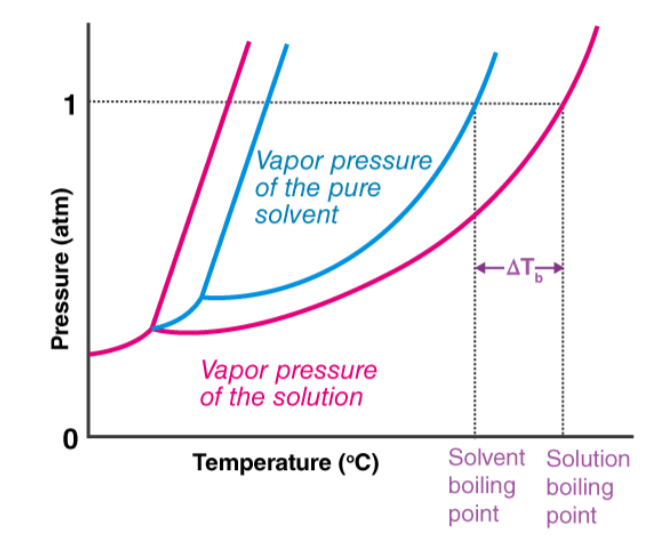 Boiling Point Elevation[/caption]
Boiling Point Elevation[/caption]
Why Does Boiling Point Elevation Occur?
The boiling point of a liquid is the temperature at which its vapor pressure is equal to the atmospheric pressure. Non-volatile liquids, with assumed zero vapor pressure, do not usually evaporate quickly. When a non-volatile solute is added to the solvent, its resulting solution has a lower vapor pressure than the pure solvent. This necessitates larger amounts of heat for the solution to boil, and this rise in boiling point is known as the boiling point elevation. A graphical analysis detailing this elevation can be observed below, where ΔTb represents the increase in boiling point. It can be seen that the freezing point of this solution is lower than that of its pure solvent (referred to as freezing point depression). Likewise, the boiling point is higher than that of its pure solvent.Also Read - Avogadro's Law Formula
Boiling Point Elevation Formula
It is possible to express the boiling point of a non-volatile solute-containing solution as follows: Boiling point of solution = pure solvent boiling point + elevation of boiling point. A solution's boiling point elevation is proportional to its solute concentration. A measurement can be made using the following equation. ΔTb = i*Kb*m, Where i is the Van’t Hoff factor. Kb is the ebullioscopic constant. m is the molality of the solute. Furthermore, this formula does not hold for volatile solvents. Alternatively, the ebullioscopic constant (Kb) is expressed in terms ofIn terms of C∘ /molal, or∘C∘kg.mol-1.Also Check - Aluminium Nitrate Formula
Below are some common solvent Kb values| Solvent | Kb Value (in Degree Celsius.kg.mol-1) |
| Water | 0.512 |
| Phenol | 3.04 |
| Acetic Acid | 3.07 |
| Chloroform | 3.63 |
| Benzene | 2.53 |
Relationship Between Boiling Point Elevation and Vapor Pressure
The concept of boiling point elevation can explain vapor pressure. It is the pressure exerted at a certain temperature by the vapor in equilibrium with its condensed phases, effectively measuring how easily the solvent molecules are able to make the transition from liquid to gas. When the vapor pressure and surrounding air pressure match, liquid boils and reaches its purest form - boiling point.What do we mean by Boiling Point Elevation?
The term "boiling point elevation" refers to the rise in the boiling point of a solvent when a non-volatile solute is added. As an example, water with salt has a higher boiling point than pure water. This phenomenon is actually a colligative property since it depends on the amount of solute present in the solution and not on its specific identity. Consequently, an increase in solute concentration will result in a more pronounced boiling point elevation.Also Check - Atomic Mass Formula
Why does Boiling Point Elevation happen?
The boiling point of a liquid is the temperature at which its vapour pressure is equal to the pressure of its nearby environment. Non-volatile substances, with their low vapour pressures assumed to be 0, do not evaporate. When a non-volatile solute is added to the solvent, the vapor pressure of the mixture decreases compared to that of the pure solvent. Consequently, more heat must be supplied for it to boil. This increase in boiling point of the solution is called boiling point elevation. In terms of vapour pressure, liquids boil when their vapor pressure equals that of their surroundings. However, when a solute is present in a solvent, it will lower its vapor pressure by dilution.Use of Boiling Point Elevation
This type of measurement is known as ebullioscopy, a Greek word for boiling viewing. It can be used to calculate the degree of dissociation or the molar mass of the solute using boiling point election and boiling point elevation formulas. According to the cryoscopic constant, the freezing point depression is larger than the ebullioscopic constant. In cryoscopy, however, the freezing point is easy to accurately measure.Boiling Point Elevation Formula FAQs
What is boiling point elevation?
How is boiling point elevation related to colligative properties?
What is the equation for calculating boiling point elevation?
Can boiling point elevation be used in everyday life?
Why does adding a solute to a solvent increase its boiling point?











