
Aluminium Nitrate Formula is an inorganic, white, solid compound composed of a cation of aluminium and the polyatomic NO3- anion. It has the chemical formula Al(N O 3 ) 3 , which may also be expressed as Mg(N O 3 ) 3 :9 H 2 O when in its hydrated form. Donating three electrons to achieve a stable electronic configuration, aluminium atoms can form a neutral salt with nitrate ions. Aluminium nitrate is, therefore, accessible both in its anhydrous and hydrated states.
Structure of Aluminium Nitrate
[caption id="attachment_14880" align="aligncenter" width="300"]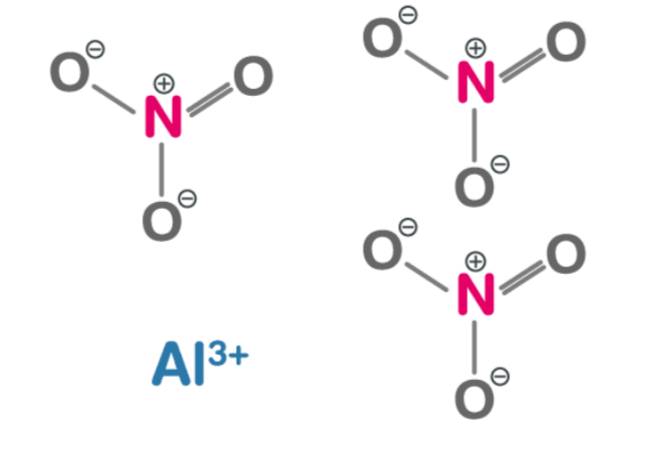 Aluminium Nitrate Structure[/caption]
Aluminium Nitrate Structure[/caption]
Aluminium Nitrate, a chemical compound with the formula Al(N O 3 ) 3 , is a white crystalline solid that embodies an intricate and fascinating structure. At first glance, it may appear as a simple arrangement of aluminum ions and nitrate groups; however, upon closer inspection, its complexity becomes apparent. The crystal lattice of aluminum nitrate forms through the attractive forces between positively charged aluminum cations (Al3+) and negatively charged nitrate anions (NO3-). These forces form octahedral coordination complexes where each aluminum ion is surrounded by six surrounding nitrate ions arranged in a distorted octahedron geometry. This unique arrangement allows efficient packing within the lattice structure while maximizing electrostatic interaction between ions. As a result, Aluminium Nitrate exhibits remarkable stability and high melting point due to ionic solid bonds holding its constituents together. Understanding this structural intricacy provides valuable insights into the behavior and properties of Aluminium Nitrate in various applications, such as catalysts or oxidizing agents in industrial processes.
Also read : Aluminum Acetate Formula
Properties of Aluminium Nitrate
In the modern periodic table, aluminium nitrate is classified as a transition metal nitrate, as aluminium belongs to Group III, the first group of transition metal elements. It is a white crystalline solid and is a salt of aluminium and nitric acid. Among its other names are nitric aluminium salt, aluminium nitrate, and aluminium (III) nitrate.
Physical Properties of Aluminium Nitrate
- The molecular mass of aluminium nitrate, as estimated from its chemical formula, is 212.996 g/mol in anhydrous form and 375.134 g/mol in nonahydrate form.
- Its formula reveals that it is a solid electrolyte and oxidizing agent. Odourless, the salt has a density of 1.72 g/cm3 in the nonahydrate form and a melting point of 66℃ for anhydrous and 73.9℃ for the nonahydrate variant. Above 150℃, the latter begins to decompose.
- Highly soluble in water, ethanol, methanol, and ethylene glycol due to ionic and structural properties listed by its formula and structural model, aluminium nitrate also exhibits hygroscopicity - its hydrated forms can retain water molecules. Flashpoint occurs at 35℃.
Download PDF Aluminium Nitrate Formula
Aluminium Nitrate Reactions and Uses
The synthesis of aluminium nitrate is generated by neutralizing dilute nitric acid with aluminium (III) chloride or an aluminium salt such as aluminium hydroxide. When a concentrated form of nitric acid is reacted with any aluminium salt, a passivation layer is produced. The by-product of this reaction is nitrosyl chloride which escapes the solution in the form of bubbles. To illustrate this production method, here is the chemical formula for aluminium nitrate/molecular formula of aluminium nitrate and other compounds involved:
3 HN O 3 ) (dilute) + AlC l 3 → Al(N O 3 ) 3 + 3HCl
A metathesis reaction is another way to produce aluminium nitrate. In the metathesis reaction, aluminum sulfate reacts with a nitrate salt of any suitable cation such as barium, strontium, calcium, silver, or lead. Below is an example of a reaction in which aluminium sulfate reacts with barium nitrate to produce aluminum nitrate. Here are the chemical and molecular formulas for aluminium nitrate:
A l 2 (S O 4 )+ 3Ba(NO3)2 3Ba(N O 3 ) → 2 Al(N O 3 ) 3 + 3BaS O 4
Aluminium nitrate salt is much rarer than its counterparts aluminium chloride and sulphate. Industry produces and relies on the former two more despite its noteworthy use. A main application of aluminium nitrate is in producing alumina which has various uses such as manufacturing insulating papers, cathode ray tube heating elements, transformer core laminates. Hydrated forms of the compound are integral in extracting actinides from the modern periodic table. Moreover, aluminium nitrate is a powerful oxidizing agent used for tanning leather, antiperspirants, corrosion inhibition, uranium extraction, and refining of petroleum products. It also acts as a nitrating agent.
In the laboratory and classroom, it is used to demonstrate the reaction:
Al(N O 3 ) 3 + 3NaOH → Al(OH)3 + 3NaNO3
Aluminium Nitrate Formula FAQs
What is the chemical formula of aluminum nitrate?
How does aluminum nitrate look?
What is aluminum nitrate's primary use?
Is aluminum nitrate a weak or strong acid?
What are the hazards of aluminum nitrate?










