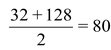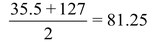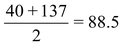Need For Classification
Periodic Classification of Class 10
One hundred and fourteen elements are known till date. Each of these elements show different properties due to different kind of atoms constituting them. So it is difficult to study the properties and uses of all the elements individually. Therefore these have been classified into groups based on similarities in their properties. These arrangements of elements are called classification of elements and this led to the formulation of a periodic table.
EARLY ATTEMPTS TO CLASSIFY ELEMENTS:
Metals and Non-Metals:
Among the earlier classifications, Lavoisier classified the elements as metals and non-metals. However, this classification proved to be inadequate. In 1803, John Dalton published a table of relative atomic weights (now called atomic masses). This formed an important basis of classification of elements.
Dobereiner's Triads:
The earliest effort concerning classification grouping of elements is that of Dobereiner in 1829. He arranged similar elements in groups of three and showed that the atomic weights are either nearly the same of the atomic weight of the middle element is approximately the arithmetic mean of the other two. These groups of three elements are called Dobereiner’s Triads.
e.g.
|
Elements of the triad |
Symbol |
Atomic mass |
|
Lithium |
Li |
7 |
|
Sodium |
Na |
23 |
|
Potassium |
K |
39 |
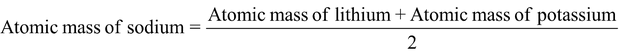
= 7 + 39/2 = 23
Some examples of triads are given in the table:
|
S.No. |
Triads |
Relative atomic masses |
Average of atomic masses of the 1st and the 3rd element |
|
1. |
S, Se, Te |
32, 79, 128 |
|
|
2. |
Cl, Br, I |
35.5, 80, 127 |
|
|
3. |
Ca, Sr, Ba |
40, 88, 137 |
|
Limitations of Dobereiner's Classification:
(a) Atomic mass of the three elements of some triads is almost same.
e.g.Fe, Co, Niand Ru, Rh, Pd
(b) It was restricted to few elements, therefore discarded.
THE TELLURIC HELIX (1862) :
It was in 1862, that a periodic classification of the elements was developed that approached the idea we have today. At that time A.E. de Chancourtois, a professor of Geology at the Ecole des Mines in Paris presented an account of his telluric helix in which he indicated the relative properties of elements and their atomic weights.
He used a vertical cylinder with 16 equidistant lines on its surface, the lines lying parallel to the axes. Then he drew a helix at 45 degree to the axis and arranged the elements on the spiral in the order of their increasing atomic weights. In this manner, elements that differed from each other in atomic weight by 16 or multiples of 16 fell very nearly on the same vertical line. In addition to the 16 vertical lines, de Chancourtois felt that other connecting lines could be drawn, and that all elements lying on such lines were related in some manner. His arrangement resulted in the proposal by de Chancourtois that the properties of the elements are the properties of numbers.
NEWLANDS’ LAW OF OCTAVES
Dobereiner’s attempt encouraged other scientists to correlate the atomic masses of the elements with their properties.
Newlands law of octaves : He observed that when the lighter elements are arranged in order of their increasing atomic weights. The properties of every eight element are similar to the first one. This generalization was named as Newlands law of Octves.
Newlands’s Octaves
|
sa (do) |
re (re) |
ga (mi) |
ma (fa) |
pa (so) |
da (la) |
ni (ti) |
|
H |
Li |
Be |
B |
C |
N |
O |
|
F |
Na |
Mg |
Al |
Si |
P |
S |
|
Cl |
K |
Ca |
Cr |
Ti |
Mn |
Fe |
|
Co and Ni |
Cu |
Zn |
Y |
In |
As |
Se |
|
Br |
Rb |
Sr |
Ce and La |
Zr |
- |
- |
In Newlands’ octaves properties of lithium and sodium were similar and sodium is the eighth element from Lithium.
LIMITATIONS:
(i) It was found that the law of octaves was applicable only upto Ca or we can say that it is applicable only for the lighter elements.
After Ca every eighth element did not possess properties similar to that of first one.
(ii) It was assumed by Newland that only 56 elements existed in nature and no more elements would be discovered in the future. But later on many more elements were discovered whose properties did not fit into the law of octaves.
(iii) In order to fit elements into his table, Newlands not only adjusted two elements into the same slot, but also put some unlike elements under the same slot.
For Example, Co and Ni are in the same slot and these are placed in the same column as Fluorine, Chlorine and Bromine which have very different properties than these elements while iron which have properties similar to that of Cobalt and Nickel has been placed in a different column.
LOTHER MEYER’S CLASSIFICATION (1869)
In 1869, Lother Meyer studied the physical properties like volume, melting point, boiling point etc. of different elements. He plotted a graph between atomic volumes for a number of elements.
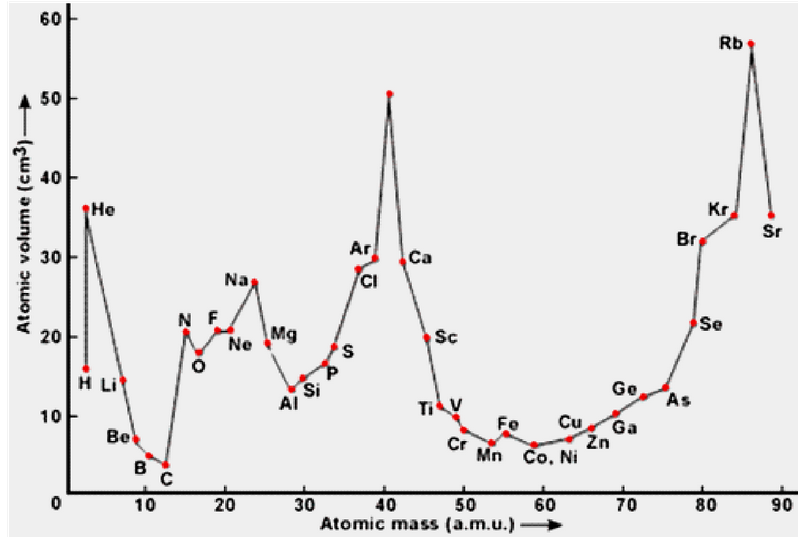
He observed that
- Strong electropositive elements except Li, all others Na, K, Rb, Cs etc. occupied the top position on the graph
- Elements Be, Mg, Ca, Sr, Ba etc. occupied the middle positions on the descending part of the graph
- Halogens occupied the middle position on ascending part of the graph.The transition elements occupy minima of curve