Classification Of Solids
Solid State of Class 12
Classification Of Solids
Solids are broadly classified into two types crystalline solids and amorphous solids.
A crystalline solid is a substance whose constituent particles possess regular orderly arrangement e.g. Sodium chloride, sucrose, diamond etc.
An amorphous solid is a substance whose constituent particles do not possess a regular orderly arrangement e.g. glass, plastics, rubber, starch, and proteins. Though amorphous solids do not possess long range regularity, in some cases they may possess small regions of orderly arrangement. These crystalline parts of an otherwise amorphous solid are known as crystallites.
An amorphous solid does not posses a sharp melting point. It undergoes liquefication over a broad range of temperature. The amorphous solid do not posses any characteristic heat of fusion. When an amorphous solid is cut with the help of sharp edged knife it results in an irregular cut.
Amorphous substances are also, sometimes, referred to as super cooled liquids because they posses disorderly arrangement like liquids. In fact many amorphous solids such as glass are capable flowing. Careful examination of the window panes of very old houses reveals that the panes are thicker at the bottom than at the top because the glass has flown under constant influence of gravity.
DISTINCTION BETWEEN CRYSTALLINE AND AMORPHOUS SOLIDS
|
Crystalline solids |
Amorphous solids |
|
1. The internal arrangement of particles is regular so they possess definite and regular geometry |
1. The internal arrangement of particles is irregular. Thus they do not have any definite geometry. |
|
2. They have sharp melting points |
2. They do not have sharp melting points |
|
3. There is regularity in the external form when crystals are formed |
3. There is no regularity in the external form when amorphous solids are formed |
|
4. Crystalline solids give a regular cut when cut with a sharp – edged knife |
4. Amorphous solids give irregular cut. |
|
5. They have characteristic heat of fusion. |
5. They do not have characteristic heat of fusion. |
|
6. Crystalline solids are rigid and their shape is not distorted by mild distorting forces |
6. Amorphous solid are not very rigid. These can be distorted by bending or compressing forces. |
|
7. Crystalline solids are regarded as true solids |
7. Amorphous solids are regarded as super cooled liquids or pseudo solids |
|
8. Crystalline solids are anisotropic. This implies that physical properties such as refractive index, conductivity, thermal expansion etc are different in different directions. This is due to orderly arrangement of particles |
8. Amorphous solids are isotropic in nature. This implies that various physical properties are same in all the directions. This is because of random arrangement of particles. |
USES OF AMORPHOUS SOLIDS
Amorphous solids such as glass and plastics are very important materials and are widely used in construction, house ware, laboratory ware etc. Amorphous silica is likely to be the best material for converting sunlight into electricity (photovoltaic). Another well known amorphous solid is rubber which is used in making tyres shoes soles etc.
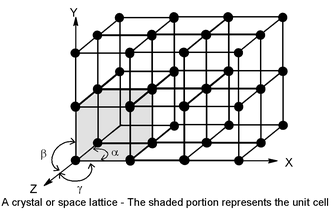
SPACE LATTICE OR CRYSTAL LATTICE
All crystals consists of regularly repeating array of atoms, molecules or ions which are the structural units (or basic units). It is much more convenient to represent each unit of pattern by a point, called lattice point, rather than drawing the entire unit of pattern. This results in a three dimensional orderly arrangement of points called a space lattice or a crystal lattice
Thus, a space lattice may be defined as a regular three dimensional arrangement of identical points in space or it can be defined as an array of points showing how molecules, atoms or ions are arranged at different sites in three dimensional space.
It must be noted that
(a) Each lattice point has the same environment as that of any other point in the lattice
(b) The constituent particles have always to be represented by a lattice point, irrespective of whether it contains a single atom or more than one atoms