
Introduction
Some Basic Concept Of Chemistry of Class 11
Chemistry is the science of substances, their properties, structures and their transformation. As all objects in the universe are made of matter. Chemistry is the branch of the science which deals with the study of material object. Study of chemistry is very interesting which covers various aspects of our culture and environment.
All development in any science are based on scientific approach as in chemistry too. In order to achieve correct results, one has to rely upon the various skills connected with the measurements of quantities during a physical or chemical change. The degree of accuracy is closely linked with precision of the measuring instrument as well as on the skill of the person engaged in measurement. So we should be first familiar with some terminology used in chemistry.
Physical Property:
The property which can be measured without changing the chemical composition of the substance known as physical property like mass, volume, density, refractive index etc.
Chemical Property:
The property which can be evaluated at the cost of matter itself known as chemical property. For example combustible nature of hydrogen gas can be verified by burning of hydrogen. The sweet taste of sugar by consuming it.
Units for Measurement
All physical quantities have to be measured. The value of a physical quantity is expressed as the product of the numerical value and the unit in which it is expressed.
Fundamental Units:
Fundamental units are those units which can neither be derived from one another nor they can be further resolved into any other units.
The seven fundamental units of measurement in S.I. system.
|
Name of unit |
Abbreviation |
|
|
Mass |
Kilogram |
Kg |
|
Length |
Meter |
m |
|
Temperature |
Kelvin |
K |
|
Amount of substance |
Mole |
Mol |
|
Time |
Second |
S |
|
Electric current |
Ampere |
A |
|
Luminous intensity |
Candela |
Cd |
Derived unit:
Some quantities are expressed as a function of more than one fundamental units known as derived units. For example velocity, acceleration, works, energy etc.
|
Quantity with Symbol |
Unit (S.I.) |
Symbol |
|
Velocity (v |
Metre per sec |
ms −1 |
|
Area (A) |
Square metre |
m 2 |
|
Volume (V) |
Cubic metre |
m 3 |
|
Density (ρ) |
Kilogram m−3 |
Kg m −3 |
|
Energy (E) |
Joule (J) |
Kg m 2 s −2 |
|
Force (F) |
Newton (N) |
Kg ms −2 |
|
Frequency (ν) |
Hertz |
Cycle per sec |
|
Pressure (P) |
Pascal (Pa) |
Nm−2 |
|
Electrical charge |
Coulomb (C) |
A-s (ampere – second) |
Units and Dimensional Analysis: Conversion of Units
The simplest way to carry out calculations that involve different units is to use dimensional analysis. In this method a quantity expressed in one unit is converted into an equivalent quantity with a different unit by using conversion factor which express the relationship between units:
Original quality×(conversion factor = equivalent quantity
(in former unit) (in other unit)
This is based on the fact that ratio of each fundamental quantity in one unit with their equivalent quantity in other unit is equal to one
For example in case of mass
1kilogram/2.205 pound = 1 = 1kilogram/1000 gm
So 1 kg = 2.205 pond = 1000 gm
In this way any derived unit first expressed in dimension and each fundamental quantities like mass length time are converted in other system of desired unit to work out the conversion factor.
For example: How unit of work / energy …………… joule in S.I. system related with unit erg in C.G.S system
Dimension of work = force × displacement = MLT-2 × L = ML2T-2
1 joule = 1 kg (1 metre) 2 × (1see)-2
⇒

⇒ 100 gm × (100) 2 × 1 em 2 × (1 sec)-2
⇒ 1000×10000 × 1 gm×1 cm 2 × 1 sec-1
⇒ 1 joule = 10 7 erg
Similarity we can deduce other conversion factor for other quantity in different unit by the dimensional analysis method
Another interesting example is the conversion of litre – atmosphere to joule (the SI unit of energy) by multiplying with two successive unit factors. Thus,
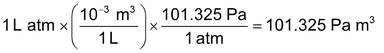
Knowing that
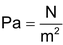 , we can write
, we can write
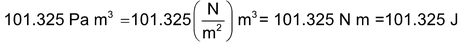
Hence 1 L atm = 101.325 J
Matter
Anything that exhibits inertia known as matter. The quantity of matter is its mass. e.g. chalk table.









