
NCERT Solutions for Class 7 Science Chapter 6
NCERT Solutions for Class 7 Science Chapter 6: This page consist of details solution of chapter Physical and Chemical Changes class 7 science .You can check NCERT Solutions for class 7 Science for other chapters of class 7 science prepared by Physics Wallah. do the questions by yourself and check your solution with Physics Wallah NCERT solution for the chapter Physical and Chemical Changes.
To have more depth in chapter Physical and Chemical Changes class 7 science you must read the theory of Physical and Chemical Changes and do the additional question of Physics Wallah click this link to do the questions and read theory of Physical and Chemical Changes. along with NCERT solution of class 7 science.CBSE Board Exam Centre List 2024
NCERT Solutions for Class 7 Science Chapter 6 Physical and Chemical Changes Overview
To understand better, students should read Chapter 6 ('Physical and Chemical Changes') of the Class 7 Science syllabus. This chapter covers essential topics, and the NCERT Solutions for Class 7 Science Chapter 6 contain valuable insights. It's advisable for students to thoroughly study each topic to grasp the chapter's concepts and effectively use the provided answers. These solutions, created by Physics Wallah's expert instructors, aim to improve understanding. The goal is to help students score well in tests by reviewing and practicing these solutions.NCERT Solutions for Class 7 Science Chapter-6 Physical and Chemical Changes
| CBSE Syllabus Class 7 | |
| CBSE Class 7 English Syllabus | CBSE Class 7 Math Syllabus |
| CBSE Class 7 Social Science Syllabus | CBSE Class 7 Science Syllabus |
NCERT Solutions for Class 7 Science Chapter 5 Exercise 1
Question 1: Classify the changes involved in the following processes as physical or chemical changes:
(a) Photosynthesis
(b) Dissolving sugar in water
(c) Burning of coal
(d) Melting of wax
(e) Beating aluminium to make aluminium foil
(f) Digestion of food
Answer: (a) Photosynthesis : Chemical change. Photosynthesis involves the conversion of carbon dioxide and water into glucose and oxygen, a transformation of substances into entirely new ones through chemical reactions.
(b) Dissolving sugar in water : Physical change. This process involves the sugar particles dispersing throughout the water but does not change the chemical makeup of sugar itself.
(c) Burning of coal : Chemical change. Burning coal involves a reaction with oxygen, resulting in the release of heat, light, carbon dioxide, and other byproducts, fundamentally altering the composition of coal.
(d) Melting of wax : Physical change. When wax melts, it changes from a solid to a liquid state without altering its chemical composition.
(e) Beating aluminum to make aluminum foil : Physical change. The process of beating aluminum alters its shape and form but doesn't change its chemical composition. However, the process of making aluminum foil involves rolling and thinning, which can be classified as a type of chemical change due to mechanical and thermal actions affecting the metal.
(f) Digestion of food : Chemical change. Digestion involves the breakdown of complex food molecules into simpler ones through chemical reactions, transforming food into nutrients that can be absorbed by the body.

Question 2: State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces is a chemical change. (True/False)
(b) Formation of manure from leaves is a physical change. (True/ False)
(c) Iron pipes coated with zinc do not get rusted easily. (True/False)
(d) Iron and rust are the same substances. (True/False)
(e) Condensation of steam is not a chemical change. (True/False)
Answer: (a) False
Cutting a log of wood into pieces is a physical change.(b) False
Formation of manure from leaves is a chemical change.(c) True
(d) False
Iron and rust are different substances. Rust is iron oxide Fe2O3(e) True
Question 3: Fill in the blanks in the following statements:
(a) When carbon dioxide is passed through lime water, it turns milky due to the formation of _________.
(b) The chemical name of baking soda is _________.
(c) Two methods by which rusting of iron can be prevented are _________ and ____ ____.
(d) Changes in which only _____ __ properties of a substance change are called physical changes.
(e) Changes in which new substances are formed are called _________changes.
Answer: (a) When carbon dioxide is passed through limewater, it turns milky due to the formation of calcium carbonate (CaCO 3 ) .
(b) The chemical name of baking soda is sodium hydrogen carbonate.
(c) Two methods by which rusting of iron can be prevented are galvanization and painting.
(d) Changes in which only physical properties of a substance change are called physical changes.
(e) Changes in which new substances are formed are called chemical changes.
Question 4: When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Answer: When baking soda (Sodium hydrogen carbonate) is mixed with lemon juice (citric acid), bubbles are formed. The bubbles are formed due to the evolution of carbon dioxide gas.
This is a chemical change. In this change, citric acid contained in lemon juice reacts with sodium hydrogen carbonate which results in the evolution of carbon dioxide gas. Lemon juice + Baking soda → Carbon dioxide + Other substances Citric acid Sodium Hydrogen carbonateQuestion 5: When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Answer: When a candle burns, both physical and chemical changes take place.
Physical change → Melting of wax Chemical change → Burning of wax Eating of food is another example where both physical and chemical changes occur simultaneously. Physical change → Breaking down of larger food particles into smaller particles Chemical change → Digestion of foodQuestion 6: How would you show that setting of curd is a chemical change?
Answer: Once the curd is formed, milk cannot be re-obtained from it. Also, both milk and curd have different properties. Since these are the properties of a chemical change, setting of curd is a chemical change.
Question 7: Explain why burning of wood and cutting it into small piece are considered as two different types of changes.
Answer: When we burn wood, a new substance, coal, is formed. Therefore, it is a chemical change. However, when we cut wood, only the shape and size of the wood are changed. No new substance is formed. Therefore, it is a physical change.
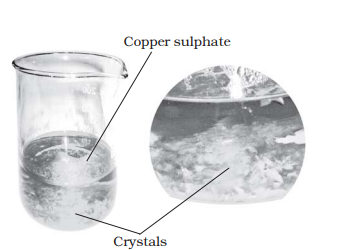
Question 8: Describe how crystals of copper sulphate are prepared.
Answer: Crystals of copper sulphate are prepared by the method of crystallization. The process is as follows. A cupful of water is taken in a beaker. Few drops of dilute sulphuric acid are added to this. The water is then heated and when it starts boiling, copper sulphate powder is added with stirring. Copper sulphate powder should be added on till the solution becomes saturated. It is then filtered into a china dish and allowed to cool. The solution should be kept undisturbed. Slowly, the crystals of copper sulphate separate out.
Question 9: Explain how painting of an iron gate prevents it from rusting.
Answer: Rusting is aided by both moisture (water) and air (oxygen). By painting an iron gate, we prevent its contact from the air and moisture present in the atmosphere. Hence, rusting is prevented.
Question 10: Explain why rusting of iron objects is faster in coastal areas than in deserts.
Answer: Both air and moisture are required for rusting to take place. In coastal areas, the quantity of moisture present in air is more than that in deserts. In desert areas, the amount of moisture in air is even lower. Therefore, rusting of iron objects is faster in coastal areas than in deserts.
Question 11: The gas we use in the kitchen is called liquefied petroleum gas (LPG). In the cylinder it exists as a liquid. When it comes out from the cylinder it becomes a gas (Change − A) then it burns (Change − B). The following statements pertain to these changes. Choose the correct one.
(i) Process − A is a chemical change.
(ii) Process − B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer: (ii) Process − B is a chemical change.
Question 12: Anaerobic bacteria digest animal waste and produce biogas (Change − A). The biogas is then burnt as fuel (Change − B). The following statements pertain to these changes. Choose the correct one.
(i) Process − A is a chemical change.
(ii) Process − B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer: (iii) Both processes A and B are chemical changes.
Extended Learning
Question 1: Describe two changes that are harmful. Explain why you consider them harmful? How can you prevent them?
Answer: (i) Rusting: If a piece of iron is open for some time, it acquires a film of brownish substance. This substance is called rust and the process is called rusting.
Rusting is harmful because it destroys the iron objects. Iron is the most widely used metal and so rusting is such a serious problem. Prevention of rusting: Rusting can be prevented by preventing iron articles from coming in contact with oxygen, or water, or both. • One simple way is to apply a coat of paint or grease. In fact, these coats should be applied regularly to prevent rusting . • Another way is galvanization, i.e., to deposit a layer of metal like chromium or zinc on iron.(ii) Spoilage of food: Food item when kept carelessly, get spoiled. This is a chemical change and obviously harmful for us.
Food is spoiled by microorganisms.Prevention of food spoilage: Microorganisms do not survive at high or low temperature. So, food items stored in refrigerator do not spoil. Also we should keep them covered so that microorganisms do not get any chance to enter and spoil them.
Question 2: Take three glass bottles with wide mouths. Label them A,B and C. Fill about half of bottle A with ordinary tap water. Fill bottle B with water which has been boiled for several minutes, to the same level as in A. In bottle C, take the same boiled water and of the amount as in other bottles. In each bottle put a few similar iron nails so that they are completely under water. Add a teaspoonful of cooking oil to the water in bottle C so it forms a film on its surface. Put the bottles away for a few days. Take out nails from each bottle and observe them. Explain your observations.
Answer:
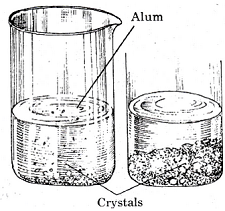
The nails in bottles B rust a little, nails in A are the most rusted and that in C remain unchanged. For rusting both water and oxygen are necessary. Both the factors are present in the bottle A since oxygen is dissolved in water. In bottle B, water is boiled and hence dissolved air is removed. Due to lack of oxygen, iron nails do not rust much. In bottle C, the layer of oil present prevents dissolving of atmospheric air in the water and hence no rusting occurs.Question 3: Prepare crystals of alum.
Answer: A cupful of water taken in beaker and a few drops of dilute sulphuric acid are added into it. The water is heated. When it starts boiling alum powder is added slowly while stirring continuously. Alum powder is added continuously till no more powder can be dissolved. The solution is filtered and allowed to cool down. Crystals of alum slowly form at the bottom of the beaker.
Question 4: Collect information about the types of fuels used for cooking in your area. Discuss with your teachers/parents/others which fuels are less polluting and why ?
Answer: The different fuels used for cooking are wood, charcoal, cow-dung cake, kerosene, biogas, LPG, etc. Among all these, biogas and LPG are least polluting. Both of these burn completely and do not give smoke. Also they do not leave any residue (ash, unburnt part, etc.)
NCERT Solutions for Class 7 Science Chapter 6 FAQs
What is a physical change? Provide examples.
Define a chemical change and provide examples.
How can you differentiate between physical and chemical changes?
Describe the process of rusting of iron and classify it as a physical or chemical change.
Explain the melting of wax as a physical change.








