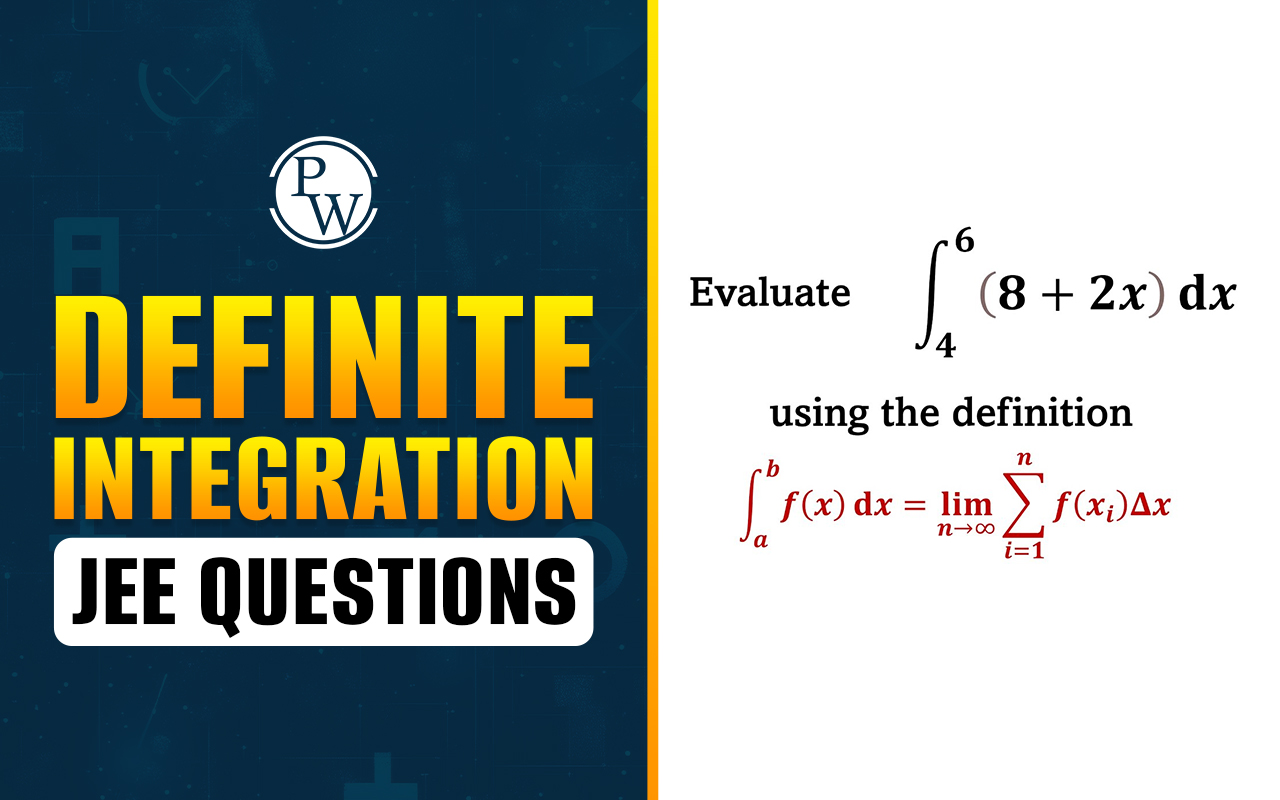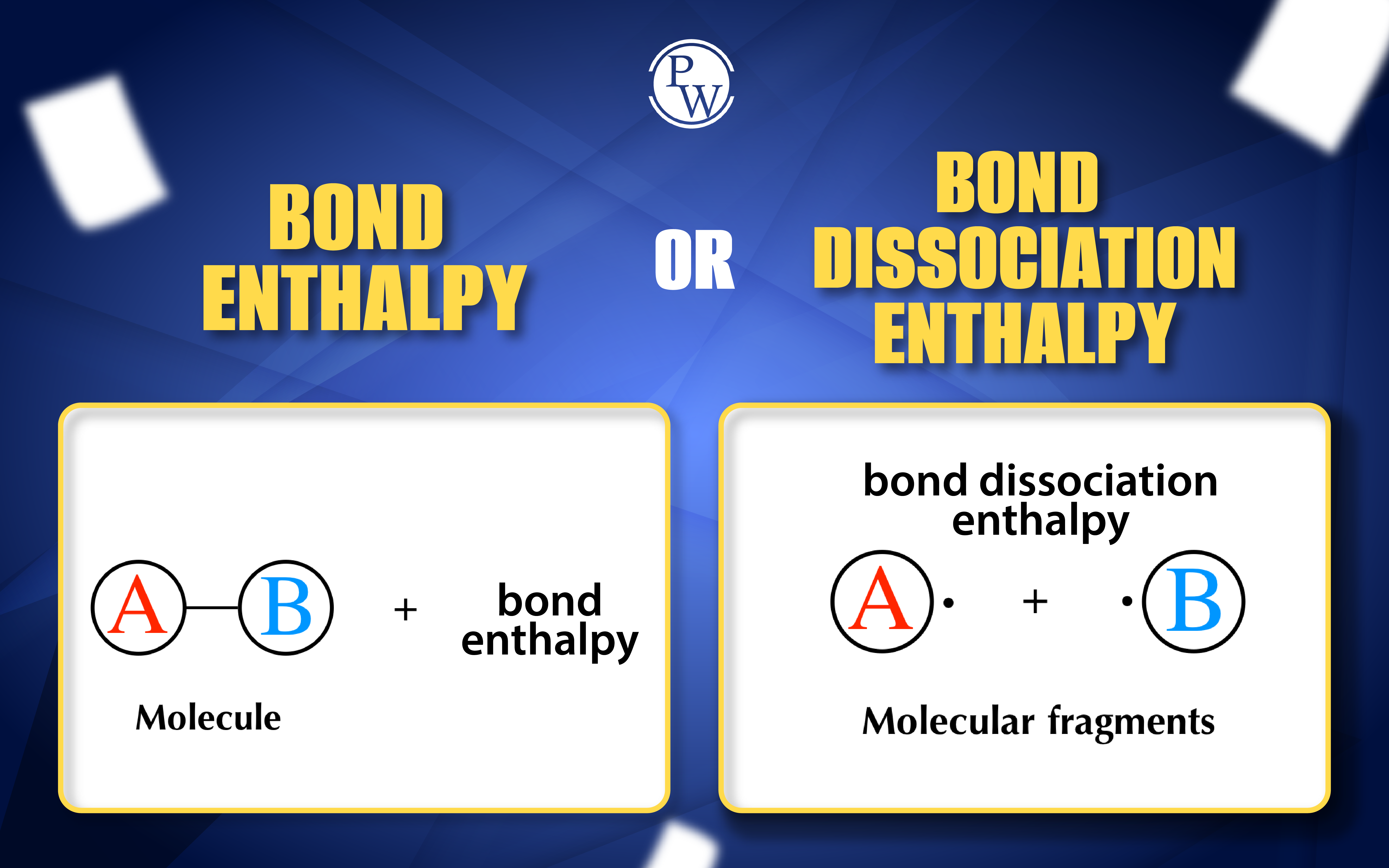
also known as bond dissociation enthalpy, a crucial factor in understanding the energetics of chemical reactions. This article delves into the depths of bond enthalpy, unraveling its mysteries and exploring its intricate relationship with various bond parameters.
Bond Enthalpy : The Energetic Tapestry of Chemical Bonds
Bond enthalpy is a measure of the energy required to break a chemical bond completely and separate the bonded atoms. It serves as a fundamental parameter in deciphering the energetic intricacies of chemical reactions. The unit of bond enthalpy is typically expressed as energy per mole, with the standard unit being kilojoules per mole (kJ/mol).How is Bond Enthalpy Experimentally Determined?
The determination of bond enthalpy involves sophisticated experimental techniques, with calorimetry being a primary method. Calorimetry allows scientists to measure the heat changes associated with a chemical reaction. In the context of bond enthalpy, this entails measuring the energy absorbed or released as bonds are broken or formed.Bond Dissociation Enthalpy: Breaking it Down
Bond dissociation enthalpy is a nuanced aspect of bond enthalpy, specifically referring to the energy required to break a particular bond in a molecule while keeping the rest of the molecule intact. To illustrate, in the case of methane (CH₄), the bond dissociation enthalpy for the C-H bond represents the energy needed to break that specific bond, leaving the other three C-H bonds unaffected.Factors Governing Bond Enthalpy
Several factors influence the bond enthalpy of a given bond, contributing to the rich tapestry of chemical interactions:Check : Important Chapters and Topics to Rank Higher In JEE
Bond Enthalpy : Bond Strength
Generally, stronger bonds exhibit higher bond enthalpies. For instance, breaking a triple bond requires more energy than breaking a double or single bond.Also Check : JEE Main Marks vs Percentile
Bond Enthalpy : Atomic Size
The size of atoms involved in bonding plays a crucial role. Smaller atoms tend to form stronger bonds due to the closer proximity of shared electrons to the nuclei, resulting in a stronger attraction.Bond Enthalpy : Multiplicity of Bonds:
Bonds with higher multiplicity (double or triple bonds) generally possess higher bond enthalpies. The sharing of more electrons in multiple bonds contributes to their increased strength.Bond Enthalpy : Bond Parameters:
Bond enthalpy provides valuable insights into the energetic aspects of chemical bonds, several other parameters contribute to a comprehensive understanding of bonding:Also Check : JEE Main Syllabus Reduced 2024
Bond Enthalpy : Bond Length
Bond length represents the average distance between the nuclei of two bonded atoms. The relationship between bond length and bond strength is inversely proportional —increasing bond length correlates with a decrease in bond strength, and vice versa.Also Check : JEE Main Marks vs Percentile
Bond Enthalpy : Bond Angle
In molecules with more than two atoms, the bond angle becomes a critical parameter. It denotes the angle between two adjacent bonds, influencing the overall geometry of the molecule and, consequently, its properties and reactivity.Also Check : JEE Main Eligibility Criteria 2024
Bond Enthalpy : Bond Order
Bond order is a numerical value that represents the strength and stability of a chemical bond within a molecule. It is a measure of the number of chemical bonds between two atoms.Check : JEE Main Chemistry Notes 2024
Bond Enthalpy : Bond Energy
Bond energy, also known as bond dissociation energy, is the amount of energy required to break a chemical bond in a gaseous state, leading to the separation of the bonded atoms. It is a measure of the strength of a bond and is expressed in energy units per mole, commonly kilojoules per mole (kJ/mol).Check : JEE Main 2024 Session 2 Registration
Bond Enthalpy : Relationship Between Bond Order and Bond Energy:
The bond energy of a molecule is directly related to its bond order. Generally, higher bond order corresponds to higher bond energy. This is because a higher bond order signifies a greater number of bonding electrons, leading to stronger electrostatic attraction between the positively charged nuclei and the negatively charged electrons.Also Check : JEE Advanced 2024 exam date
Bond Enthalpy : Bond Polarity
Differences in electronegativity between atoms in a bond give rise to bond polarity. A polar bond features an uneven distribution of electrons, leading to the development of partial positive and negative charges on the bonded atoms.Also Check : JEE Main Previous Year Question Papers
Bond Enthalpy : FAQs
Q.1: How does bond enthalpy impact the spontaneity of a reaction?
Q.2: Can bond enthalpy predict the nature of bonds formed in a compound?
Q.3: Why do multiple bonds exhibit higher enthalpies than single bonds?
Q.4: How does bond length influence bond strength?
Q.5: Can bond parameters be used to predict the geometry of molecules?
Q.6 : What does a bond order of zero signify?