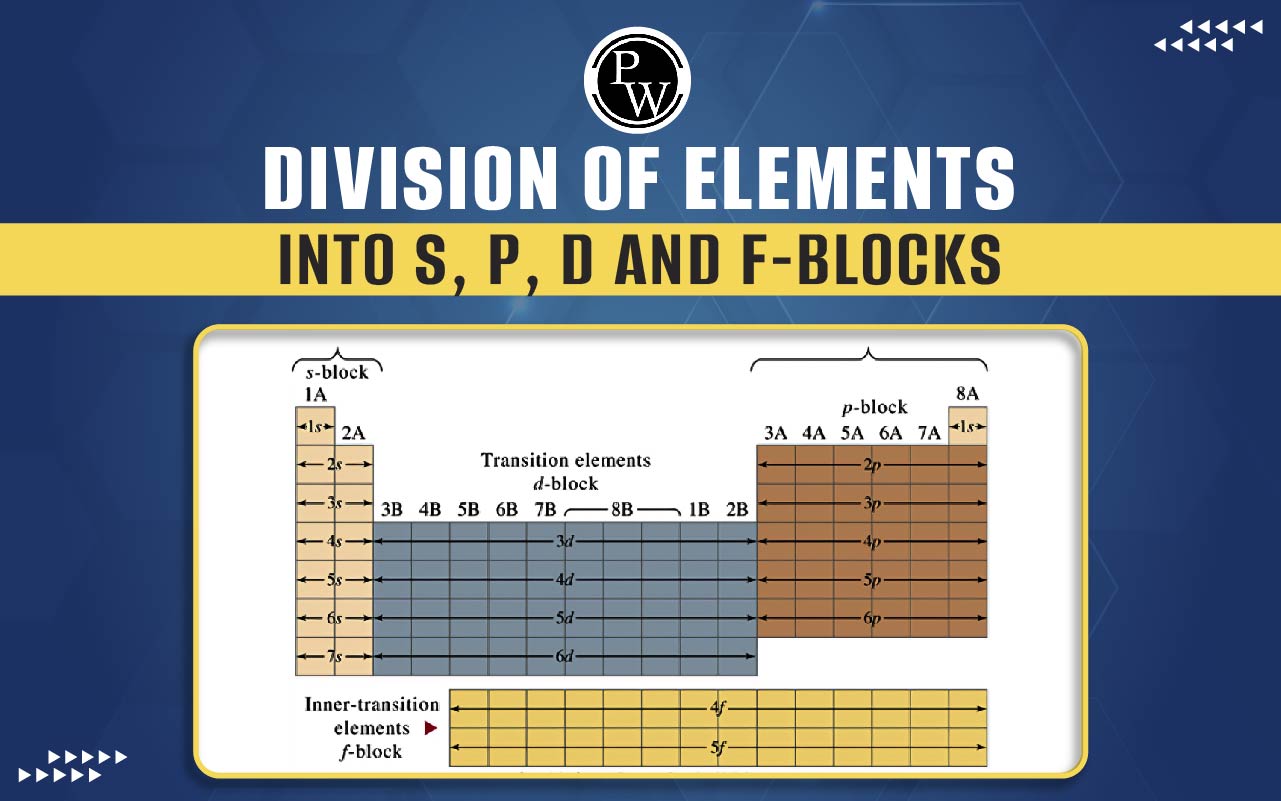
Division Of Elements Into S,P,D And F-Blocks : The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and electronic configuration. Elements are categorized into distinct blocks, namely s, p, d, and f-blocks, each with unique characteristics and properties.
S-Block Elements : The s-block encompasses two groups, Group 1 st (alkali metals) and Group 2 nd (alkaline earth metals). These elements occupy the leftmost columns of the periodic table. s-block elements are characterized by their tendency to lose electrons, resulting in the formation of positively charged ions. Alkali metals are highly reactive, especially with water, while alkaline earth metals display similar reactivity but to a lesser extent.
Read More - JEE Main Test Series: Your Path to Scoring High in the Exam
-
The elements of the periodic table in which the last electron enters in s-orbital, are s-block elements.
-
s-orbital can accommodate a maximum of two electrons.
-
Their general electronic configuration is ns 1–2 , where n = 1 to 7
1 st group elements are known as alkali metals because they react with water to form alkali. 2 nd group elements are known as alkaline earth metals because their oxides react with water to form alkali and these are found in the soil or earth crust.
-
Fr 87 and Ra 88 are radioactive elements while H and He are gaseous elements.
P-Block Elements
P-Block Elements : The p-block consists of six groups (Group 13 to Group 18) and is found on the right side of the periodic table. p-block elements display a wide range of properties. Group 17, known as the halogens, are highly reactive nonmetals, while Group 18, are noble gases, are inert and have a full complement of electrons in their outermost shell. p-block elements often form covalent bonds and exhibit diverse oxidation states.
-
The elements of the periodic table in which the last electron gets filled up in the p-orbital, called p-block elements.
-
The general electronic configuration of p-block element is ns 2 , np 1–6 (where n = 2 to 6).
-
p-subshell can accommodate a maximum of six electrons. Therefore, p-block elements are divided into six groups which are IIIA, IVA, VA, VIA, VIIA and zero group or 13, 14, 15, 16, 17 & 18.
-
The zero group (18 th ) elements having general electronic configuration ns 2 , np 6 are inert, because their octets are complete. And element belongs to this group are highly stable due to fully filled orbital.
- Group 15 elements are called “Pnicogens”
-
Group 16 elements are called “Chalcogens”
-
Group 17 elements are called “Halogens”
D-Block Elements : The d-block, also called the transition metal elements, occupies the central portion of the periodic table. These elements are characterized by the filling of the d orbitals in their electron configuration. Transition metals often display multiple oxidation states and form colourful compounds due to the presence of partially filled d-orbitals. They are known for their high melting points, conductivity, and catalytic properties. Transition metals are crucial in industrial processes.
(a) The elements, in which the last electron enters into (n – 1) d-orbital are called d-block elements
(b) The general electronic configuration of these elements is (n –1) d 1-10 , ns 0–2 where n = 4 to 7.
(c) The d-block elements are placed in the groups 3, 4, 5, 6, 7, 8, 9, 10, 11 & 12.
(d) All of these elements are metals.
(e) d-Block elements lies between s & p block elements.
F-Block Elements : The f-block includes the lanthanides and actinides, which are placed at the bottom of the periodic table. The lanthanides (rare earth elements) are notable for their similar chemical properties and are commonly used in various technologies, such as electronics and magnets. The actinides, on the other hand, are known for their radioactivity. Uranium and thorium, members of the actinide series, have significant importance in nuclear energy.
(a) The elements in which the last electron enters into (n–2) f-orbital are called f-block elements.
(b) The f-block elements are form atomic number 58 to 71 and form 90 to 103. The general electronic configuration of these elements is (n–2) f 1–14 , (n–1) d 0–1 , ns 2 where n = 6 to 7.
(c) The elements from atomic number 58 to 71 are called lanthanides because they come after lanthanum (57). The elements from 90 to 103 are called actinides because they come after actinium (89).
(d) The lanthanides occur in nature in low abundance and therefore, these are called rare earth elements.
(e) All the actinide elements are radioactive.
(f) All the elements after atomic number 92 (i.e. U 92 ) are transuranic elements.
Division Of Elements FAQs
Q.1: What are the key characteristics of s-block elements?
Q.2 : How do p-block elements differ from other element groups?
Q.3 : What distinguishes d-block elements from other elements?
Q.4 : What is the significance of f-block elements in the periodic table?
Q.5 : How does the periodic table serve as a guide for chemists?