
Electronegativity In JEE Chemistry : Electronegativity is a fundamental concept in chemistry that describes the ability of an atom to attract shared electrons in a chemical bond. It plays a crucial role in understanding various chemical phenomena, including bond polarity, molecular structure, and reactivity. This article explores the applications and different scales of electronegativity, shedding light on its significance in the realm of chemistry.
Electronegativity In JEE Chemistry : Electronegativity is a measure of the tendency of an atom to attract electrons towards itself when participating in a chemical bond. It is influenced by several factors, including atomic size, nuclear charge, and electron shielding. The concept was first introduced by Linus Pauling in 1932, and since then, various scales and methods have been developed to quantify electronegativity.
A polar covalent bond of A-B may be broken as,
(Electronegativity A> Electronegativity B)
depending on their tendency to attract bonded electron.
Important Factors Affecting Electronegativity
-
-
Atomic size
-
Ex. F>Cl>Br>I
-
-
Effective Nuclear Charge (Z eff )
-
Ex. Mn +2 <Mn +4 <Mn +7
O -2 <O -1 <O<O +1 <O +2
Fe<Fe +2 <Fe +3
-
-
% s –Character
-
Electronegativity Variation in Group and Period
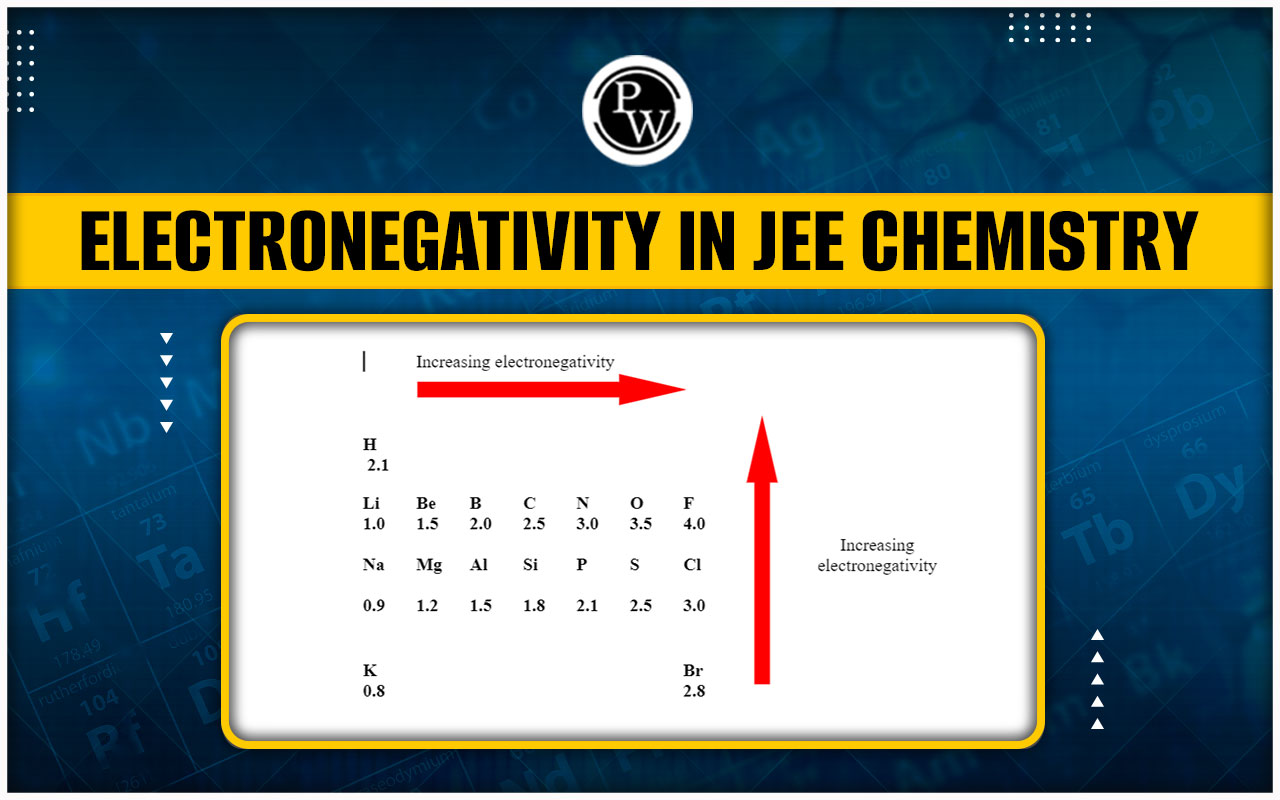
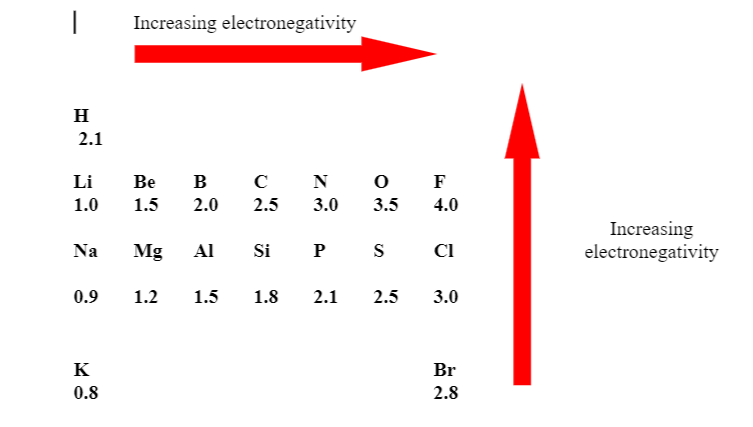
(A) Electronegativity decreases down the group.
(B) In period on moving from left to right electronegativity increases regularly.
(C) Electronegativity of Cs and Fr are equal, it is because from 55 Cs to 87 Fr only one shell increases but nuclear charge (No. of proton) increases by +32 , so effect of nuclear charge balanced the effect of increase in number of shell.
Electronegativity of F > Cl but Electron affinity of Cl > F
-
In IIIA group, value of electronegativity is irregular when going down the group, because of transition contraction.
Electronegativity of Ga > Electronegativity of Al
Electronegativity values of Some Common Elements
Applications of Electronegativity
- Bond Strength : If the electronegativity difference of covalently bonded atoms ( ΔX ) increases, the bond energy of the covalent bond also increases. For example the order of the H-X bond strength is
H – F > H – Cl > H – Br > H – I
As the bond strength decreases, the acidic strength increases.
Order of increasing acidic strength is:-
HF < HCl < HBr < H – I
- Metallic and non-metallic properties of elements :
-
The metallic character decreases as the electronegativity of the element increases.
-
On moving from left to right in a period, the electronegativity of the elements increases. So the metallic character decreases.
-
On moving down a group, the electronegativity of the elements decreases, so the metallic character increases.
- Schomaker and Stevenson law : If in a diatomic molecule electronegativities difference of A – B is more then actual bond length will be reduced. As per Schomaker and Stevenson- The reduction in bond length depends on the difference in electronegativities of atoms in following manner –
(Here X A is E.N. of A &X B is E.N. of B)
- Acidic & Basic Strength: Nature of Hydrides:
-
Stability of Molecule
Bond Energy (Strength)
Order of stability of hydro halides HF > HCl > HBr > HI
Order of acidic strength HF < HCl < HBr < HI
In VA group NH 3 < PH 3 < AsH 3 < SbH 3 < BiH 3
Thermal stability decreases
Acidic character increases
- Nature Stability Decreases:
As per Gallis, In AOH if electronegativity of A is more than 1.7 (Non metal) then it is acidic in nature.
Important Points For Electronegativity
BeO,Al 2 O 3 ZnO,SnO,PbO,SnO 2 ,PbO 2 ,Sb 2 O 3 etc. are Amphoteric oxides.
CO,H 2 O,NO,N 2 O etc. are Neutral oxides.

Electronegativity In JEE Chemistry FAQs
Q.1 : What is electronegativity, and why is it important in chemistry?
Q.2 : How is electronegativity used to determine bond polarity?
Q.3 : How does electronegativity vary across different groups and periods of the periodic table?












