
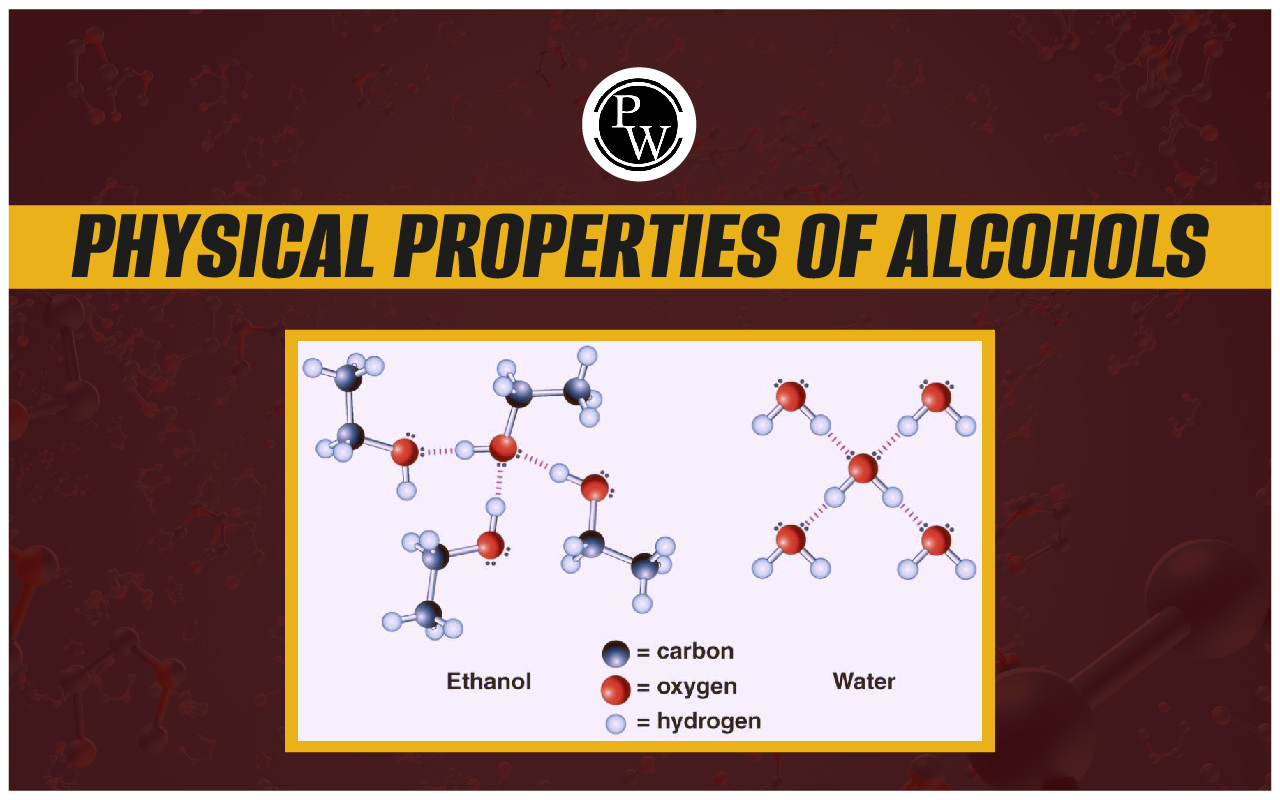
Physical Properties Of Alcohols : Alcohol molecules are used in many different contexts, including daily life and industry. Let's examine a few of alcohol's most significant physical characteristics. We will also discuss how alcohol types can be distinguished using these characteristics.
At room temperature, the majority of common alcohols are colourless liquids. Free-flowing liquids with fruity odours are methyl alcohol, ethyl alcohol, and isopropyl alcohol. Higher alcohols—those with 4 to 10 carbon atoms—are more viscous, or oily, and have stronger fruity odours. At room temperature, some highly branched alcohols and many alcohols with more than 12 carbon atoms are solids.
Physical properties Of Alcohols
Physical properties : The size and structure of the molecule determines a wide range of the physical characteristics of alcohols.
-
In general, alcohols have higher boiling points than comparable molecules without the hydroxyl group and are less volatile than water. Alcohol molecules form hydrogen bonds with one another, which causes it to happen.
-
Another significant physical characteristic of alcohol is its solubility. Alcohols can dissolve in water and other polar solvents because they are polar molecules as well.
-
Compared to other molecular compounds with similar structures, they usually have lower melting and boiling points and are less dense than water.
-
The physical characteristics of alcohol are also influenced by the molecular weight and degree of branching.
Physical state
Physical state: Lower alcohols are colourless liquids at normal temperature. The higher alcohols are colourless, odourless waxy solids.
Lower alcohol: Ethanol Higher alcohol: Cetyl Alcohol
Boiling Point
1. Alcohols have a higher boiling point than corresponding aliphatic hydrocarbons, haloalkanes, aldehydes, and ketones due to intermolecular hydrogen bonding.
2. Branching causes a decrease in surface area, which lowers the boiling point of isomeric alcohols.
CH 3 CH 2 CH 2 CH 2 OH > (CH 3 ) 2 CHCH 2 OH > (CH 3 ) 3 COH
3. The boiling point of alcohol increases with their molecular masses.
Physical properties Of Alcohols Examples
Arrange the following alcohols in the decreasing order of their boiling points.
1. n-Butyl alcohol
2. sec-Butyl alcohol
3. tert-Butyl alcohol
Explanation Of Physical properties Of Alcohols
The decreasing order of boiling points is:
n – Butyl alcohol > sec-Butyl alcohol > t-Butyl alcohol
H-bonding occurs in all alcohols, but alcohol (1) has a larger surface area. Because of branching, alcohol (2) has less surface area, whereas alcohol (3) has more branching and less surface area than alcohol (2). As a result, the boiling point order is as shown above.
|
Formula |
Name |
Molar Mass |
Boiling Point ( o C) |
|
CH 4 |
Methane |
16 |
–164 |
|
HOH |
Water |
18 |
100 |
|
C 2 H 6 |
Ethane |
30 |
–89 |
|
CH 3 OH |
Methanol |
32 |
65 |
|
C 3 H 6 |
Propane |
44 |
–42 |
|
CH 3 CH 2 OH |
Ethanol |
46 |
78 |
|
C 4 H 10 |
Butane |
58 |
–1 |
|
CH 3 CH 2 CH 2 OH |
1–propanol |
60 |
97 |
Solubility
1. Alcohols are soluble in water because they form intermolecular hydrogen bonding with water molecules.
2. The solubility decreases with increase in mass because the hydrocarbon part becomes larger and resists the
formation of hydrogen bonds with water molecules.
3. The solubility of isomeric alcohols increases with branching because the surface area of the hydrocarbon part decreases with branching.
Solubility: Primary < Secondary < Tertiary.
Example Alcohols are more soluble in water than comparable molecular mass hydrocarbons. Explain this point.
Solubility : Alcohols have a tendency to bind to water molecules and create new H-bonds when they do so. They dissolve in water as a result. Hydrocarbons, on the other hand, cannot form H-bonds; hence, they are insoluble in water.
The acidity of Alcohols
The acidity of Alcohols : When alcohols react with active metals like potassium, sodium, etc., the corresponding alkoxide is formed. These alcohol reactions show that they are acidic. Because of the polarity of the -OH bond, alcohol has an acidic nature. When an electron-donating group is bonded to the hydroxyl group, the oxygen atoms electron density increases, and the acidity of alcohols decreases accordingly. As a result, primary alcohols typically have a higher acidity than secondary and tertiary alcohols. Alcohols also function as Bronsted bases because they contain unshared electrons on the oxygen atom.
Flammability
Flammability : Many alcohols are extremely flammable. Because of their high flammability limits, methanol and ethyl alcohol are particularly hazardous. Polyols are generally flammable. Because of their low volatility, they are not easily flammable.
Physical Properties of Alcohols FAQs
Q.1 : Do alcohols form H-bonding?
Q.2 : Due to branching alcohols boiling point decreases?
Q.3 Is ethanol acidic?
Q.4: Alcohols are highly soluble in water due to?
Q.5 What is the solubility order of primary, secondary, and tertiary alcohols?












