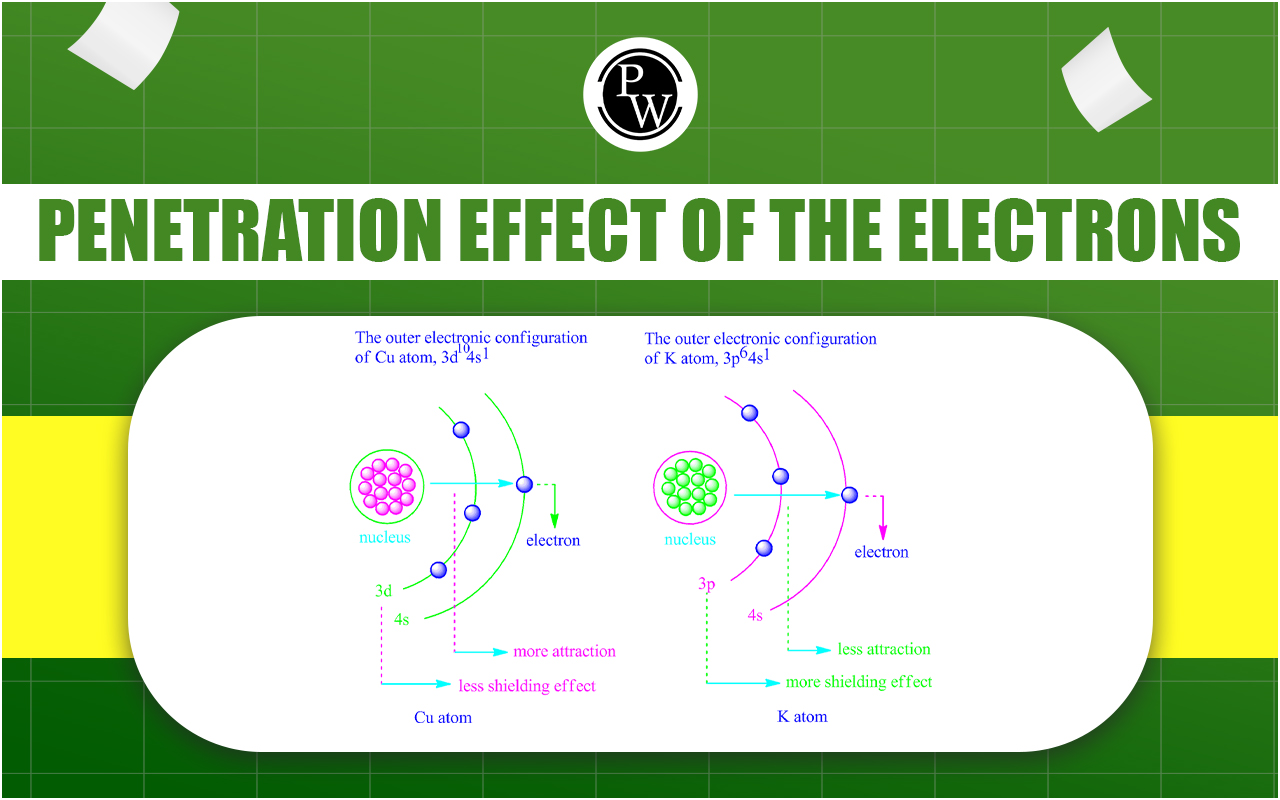
Penetration Effect Of The Electrons In Different Orbitals : In the realm of chemistry, the behaviour of electrons within atoms is a fundamental aspect that governs various chemical and physical properties. One of the key concepts in understanding electron behaviour is the penetration effect, which refers to the ability of electrons to penetrate the nucleus in different atomic orbitals. This phenomenon has significant implications in explaining various chemical phenomena and finding applications in diverse fields.
Penetration Effect of the Electrons
Penetration Effect of the Electrons: In atomic orbitals, electrons are arranged in various energy levels known as shells and subshells. These orbitals have different shapes and sizes, with some orbitals having higher probability densities closer to the nucleus, while others are spread out further away.
Penetration power is the attracting capacity of any orbital to the electron. This phenomenon or process is related to releasing energy. s-orbital has maximum penetration effect because, when an additional electron enters in the atom then, it enters in s-orbital penetrating atom. Since, s-orbital because of being nearest to the nucleus of atom so, nucleus has maximum attraction on that electron and it releases maximum energy, whereby value of electron affinity will be maximum for that electron.
Orbital Penetration Effect of the Electrons
-
Penetration describes the proximity of electrons in an orbital to the nucleus.
-
Electrons which experience greater penetration experience less shielding and therefore experience a larger Effective Nuclear Charge .
-
In a multi-electron system, the penetration of the nucleus by an electron is measured by the relative electron density near the nucleus of an atom for each shell and subshell of an electron.
-
Therefore, for the same shell value (n) the penetrating power of an electron follows this trend in subshells: s > p > d > f
-
Orbital penetration refers to how effectively electrons can get close to the nucleus.
-
For example, the electron probability density for s-orbitals is highest in the center of the orbital, or at the nucleus.
Orbital Penetration Effect of the Electrons(On the Periodic Table)
- For one principal energy level the penetrating power of an electron follows this trend in subshells:
s > p > d > f
- And overall, the penetrating power of an electron follows this trend
Exceptions of Orbital Penetration
The outer energy levels penetrate the inner levels so the shielding of the core electrons is not totally effective s > p > d > f (within the same energy level)Penetration Effect of the Electrons Application in Chemical Bonding
The penetration effect plays a crucial role in explaining the strength and nature of chemical bonds. For instance, in covalent bonds, the overlap of atomic orbitals leads to the formation of molecular orbitals. Orbitals with higher penetration, such as the 2s and 2p orbitals compared to the 1s orbital, contribute more effectively to bonding, resulting in stronger bonds. This is exemplified in molecules like water, where the oxygen atoms 2s and 2p orbitals penetrate towards the hydrogen atoms, facilitating stronger O-H bonds.
Penetration Effect of the Electrons FAQs
Q.1 : What is the Penetration Effect?
Q. 2 : How does the Penetration Effect occur?
Q.3 : Which orbitals exhibit higher penetration?
Q.4 : How does the Penetration Effect affect chemical reactivity?
Q.5 : Does the Penetration Effect have applications beyond chemistry?
Q.6 : How is the Penetration Effect related to transition metal chemistry?