Electrophilic Aromatic Substitution NEET Mindmap is important to be prepared well, as it is an important chapter in NEET Chemistry. This chapter forms the base of aromatic chemistry. Students must focus on understanding the substitution mechanisms, reaction types, and directing effects for both theory and problem-solving questions.
Electrophilic Aromatic Substitution NEET Mindmap makes this complex topic by representing it in a visual format, including all reactions, mechanisms, and key reagents. Electrophilic Aromatic Substitution NEET mindmap PDF consolidates the entire chapter into a single-page visual format. This is helpful for students to recall mechanisms, formulas, and high-yield reactions easily.
Electrophilic Aromatic Substitution NEET Mindmap Notes
Mindmap notes cover all essential aspects of EAS for NEET aspirants. To make revision easier and more effective, the table format of Electrophilic Aromatic Substitution NEET Mindmap Notes organizes all key concepts, reaction mechanisms, examples, and directing effects:
|
Electrophilic Aromatic Substitution NEET Mindmap Notes |
||
|
Concept / Reaction |
Key Point |
Example |
|
Basics |
Benzene prefers substitution (EAS) to retain aromaticity. |
Unlike alkenes, benzene avoids addition. |
|
Mechanism |
1. Electrophile generation → 2. Attack on ring (σ-complex) → 3. Deprotonation → aromaticity restored. |
Common electrophiles: NO₂⁺, SO₃, R⁺, X⁺ |
|
Nitration |
HNO₃ + H₂SO₄ → NO₂⁺ (electrophile) |
Benzene → Nitrobenzene |
|
Halogenation |
X₂ + FeX₃ → X⁺ |
Benzene → Halobenzene |
|
Sulfonation |
SO₃/H₂SO₄ → –SO₃H group |
Benzene → Benzenesulfonic acid |
|
Friedel–Crafts Alkylation |
RCl + AlCl₃ → R⁺ |
Benzene → Alkylbenzene |
|
Friedel–Crafts Acylation |
RCOCl + AlCl₃ → RCO⁺ |
Benzene → Acylbenzene |
|
Directing Effects |
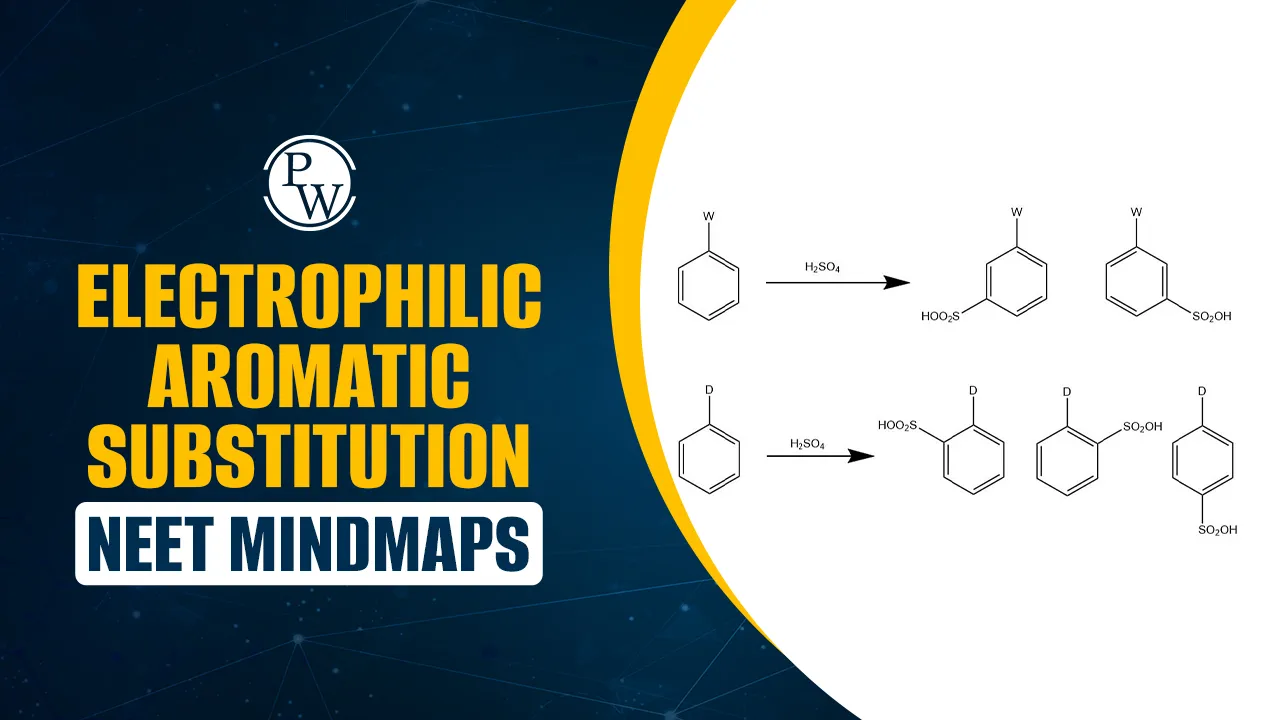
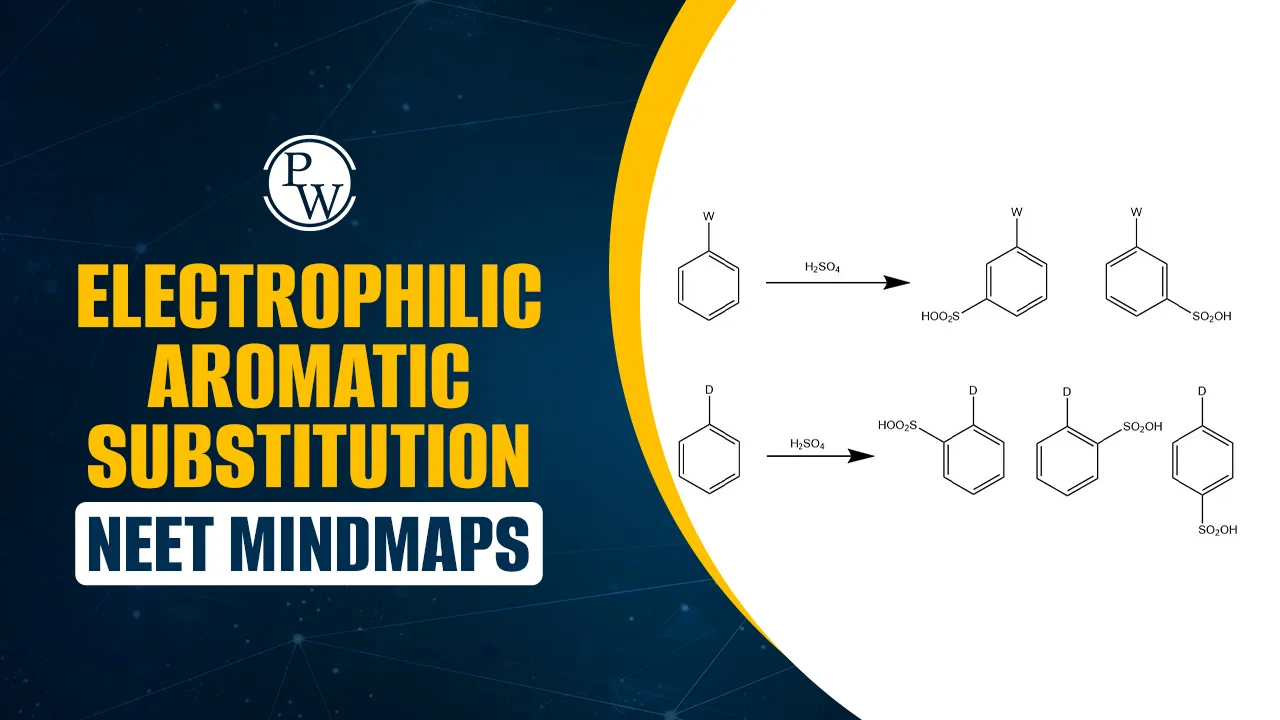
–OH, –NH₂, –OCH₃, –R → Ortho/Para (activating). –NO₂, –COOH, –CN → Meta (deactivating). |
Predicts substitution position. |
Electrophilic Aromatic Substitution NEET Mindmap PDF
Electrophilic Aromatic Substitution NEET mindmap PDF combines the entire chapter into a one-page revision tool. It covers all reaction types, mechanisms, reagents, and directing effects clearly.
You can quickly revise activating/deactivating groups, ortho/para/meta directing effects, and key reaction steps without flipping through lengthy notes. Electrophilic Aromatic Substitution NEET Mindmap PDF is useful for last-minute preparation, helping NEET aspirants recall reactions and mechanisms in seconds.
Electrophilic Aromatic Substitution NEET Mindmap PDF
Electrophilic Aromatic Substitution NEET Mindmap Formulas
Using the mindmap to link electrophile generation, attack, and deprotonation steps will help you recall mechanisms during exams. Formulas and quick references are important for solving NEET questions efficiently. Below are the Electrophilic Aromatic Substitution NEET Mindmap Formulas:
|
Electrophilic Aromatic Substitution NEET Mindmap Formulas |
|
|
Concept |
Formula / Reference |
|
Nitration |
HNO₃ + H₂SO₄ → NO₂⁺ + HSO₄⁻ + H₂O |
|
Halogenation |
X₂ + FeX₃ → X⁺ + FeX₄⁻ |
|
Sulfonation |
SO₃ + H₂SO₄ → HSO₃⁺ + HSO₄⁻ |
|
Friedel-Crafts Alkylation |
RCl + AlCl₃ → R⁺ + AlCl₄⁻ |
|
Friedel-Crafts Acylation |
RCOCl + AlCl₃ → RCO⁺ + AlCl₄⁻ |
Electrophilic Aromatic Substitution NEET Mindmap Practice Questions
Practice is key to preparing well for the EAS concept. Here are Electrophilic Aromatic Substitution NEET mindmap practice questions you should prepare well:
-
Predict the major product in substituted benzene reactions.
-
Determine the directing effect of a given substituent.
-
Solve nitration, halogenation, sulfonation, alkylation, and acylation problems.
-
Identify the position of substitution (ortho, meta, para) based on substituents.
-
Solve mechanism-based multiple-choice questions.
Mindmaps visually link mechanisms, reagents, and directing effects, making it easier to tackle both theory and numerical NEET questions.
Tricks to Revise with Electrophilic Aromatic Substitution NEET Mindmaps
Revising EAS effectively requires linking theory with patterns of aromatic reactivity. Here are some tips to revise the Electrophilic Aromatic Substitution concept with the help of the PW NEET mindmap:
-
Categorize reactions by type, i.e., Group nitration, halogenation, sulfonation, alkylation, and acylation separately with their electrophiles.
-
Use different colors for activating (ortho/para) and deactivating (meta) groups to recall substitution positions.
-
Memorize EAS by breaking it into three steps, i.e., electrophile generation then attack on the aromatic ring and then deprotonation to restore aromaticity.
-
Write down the unusual cases, like deactivated rings that undergo substitution slowly or poly-substitution patterns.
-
Instead of memorizing the concept, understand how electron-donating or withdrawing groups affect reactivity. This way you can predict products in new questions.