
Titration Formula: Titration, a method in analytical chemistry, involves the gradual addition of a known concentration solution (referred to as a titrant) to a known volume of an unknown concentration solution until the reaction reaches a state of equilibrium, often indicated by a visible color change. The titrant solution must meet specific criteria to be designated as either a primary or secondary standard. Titration is a fundamental technique for determining the concentration of an unknown solution in a general manner.
Also Check – Molecular Speed Formula
Definition of Titration
Titration is a laboratory procedure used to ascertain the concentration of an unknown solution by utilizing a solution with a known concentration.
Titration, also called titrimetry, stands as a vital qualitative analytical technique in chemistry for quantifying the concentration of a substance within a mixture. It is frequently referred to as Volumetric Analysis.
Titration Formula
The formula for titration can be expressed as follows:
% Acid = (N × V × Equivalent Weight) × 100 / (W × 1000)
Where:
N signifies the Normality of the Titrant
V stands for the volume of the titrant
While W symbolizes the mass of the sample.
Equivalent Weight indicates the equivalent weight of the acid
Also Check – Mole Fraction Formula
Titration Procedure
The titration process commences by preparing a titrant or titrator, which is a standardized solution characterized by a predetermined volume and concentration. This titrant is then allowed to interact with the analyte until an endpoint or equivalence point is achieved. At this juncture, the analyte's concentration can be approximated by measuring the quantity of titrant consumed. Titration, in essence, is a stoichiometric method employed to ascertain the unknown concentration of a solution.
In terms of procedural steps, a precise quantity of the analyte is introduced into a beaker or an Erlenmeyer flask. A small portion of the titrant, typically containing an indicator like phenolphthalein, is positioned beneath a calibrated burette or chemical pipetting syringe.
Gradually, small increments of the titrant are introduced into the analyte mixture along with the indicator. This process continues until the indicator's color change signals the saturation threshold of the titrant. At this juncture, it indicates the conclusion of the titration. Essentially, the quantity of titrant present during the reaction equals the quantity of analyte present in the solution.
Also Check – Vapor Pressure Formula
Preparation Techniques
For both the titrant and the analyte, it is essential that they exist in liquid form, typically as solutions. Solvents like glacial acetic acid or ethanol are commonly employed to dissolve solid substances. Furthermore, in the case of highly concentrated analytes, dilution is often performed to enhance precision. In many non-acid–base titrations, maintaining a stable pH throughout the reaction is crucial, and this is achieved by introducing a buffer solution into the titration vessel.
In certain situations within the reaction vessel, a separate masking solution may be introduced to neutralize the impact of undesired ions. To expedite certain redox reactions, it may be necessary to heat the sample solution and carry out the titration while it remains in a heated state.
Also Check – Theoretical Yield Formula
Chemical Analysis
Chemical analysis involves the determination of chemical components within a substance, including both their identity and quantity. This form of analysis can be categorized into two main types:
Qualitative Analysis: This focuses on identifying the various chemical species present in a compound.
Quantitative Analysis: This involves measuring the quantities of chemical species present in a compound.
Titration is a quantitative chemical analysis technique utilized to ascertain the concentration of a specific chemical species in a solution. The endpoint of a titration can be determined using various methods, including:
- Gravimetric Analysis
- Volumetric Analysis
- Spectroscopy
- Combustion
To successfully determine an unknown concentration through titration, a strong grasp of fundamental concepts such as the mole concept for balanced chemical equations and equivalence concepts for unbalanced chemical reactions is essential.
Also Check – Degree Of Unsaturation Formula
Titration Formula
A titration is a method used to determine the volume of one solution necessary to react precisely with a specified volume of another solution. Titration encompasses various types of reactions, including redox reactions and precipitation reactions, which differ from acid-base reactions.
"In a titration, the objective is to establish the exact volume of one solution needed to react completely with a known volume of a different solution."
The Titration Formula is expressed as follows:
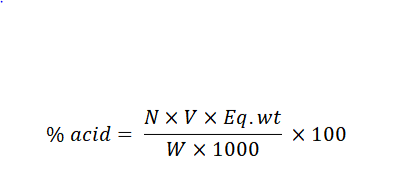
Where,
1000 = conversion factor for milligrams to grams
W = mass of the sample
N = normality of the titrant
V = volume of the titrant
Eq.wt = equivalent weight of the acid
Titration Formula Solved Examples
Example 1: Calculate the titratable acidity, expressed as the percentage of citric acid, if 17.5 mL of 0.085N NaOH is required to titrate a 15 mL sample of juice (molecular weight = 192; equivalent weight = 64).
Solution:
We can use the equation:
titration_formula
% of acid = 0.085 × 17.5 × 64 / 15 × 10 = 0.635%
It's important to note that the equivalent weight of anhydrous citric acid is always used when calculating and reporting the results of a titration.
Titration Formula FAQs
. What is titration?
What is the purpose of titration?
What is the titration formula?
What is "Normality" in titration?
Why is an indicator used in titration?










