
ICSE Worksheet for chapter-10 Nitric Acid class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of ICSE Board Worksheet for Class 10 Chemistry . Students of Class 10 Chemistry can get a free Worksheet for Class 10 Chemistry in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Standard 10 students can practice questions and answers which are given here for Chemistry in Grade 10 that will help them to improve their knowledge of all important chapters and their topics. Students can also download free pdf of Class 10 Chemistry Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
If any students need to take the online test to check their concepts or undertstanding then they can visit Chemistry Quiz for Class 10 .
Summary
- Introduction, laboratory preparation from a mixture of potassium or sodium nitrate with conc.H 2 SO 4.
- Manufacture of nitric acid by Ostwald’s process.
- Physical and Chemical properties, tests and uses of nitric acid.
Section - 1
-
Q1. Which metal nitrates give nitrogen oxide, oxygen and metal oxide, on heating?
Which metal nitrates gives their metal on heating? -
Q2. Answer the questions given below, relating your answer only to compounds given in the following lists:
Tetrammine copper (II) sulphate , Iron (III) chloride, conc.Nitric acid,
Ammonium hydroxide.- Write name of the colours and action when concentrated nitric acid is heated with copper turnings?
- A compound X is dissolved in the ammonia solution to give a deep blue colored solution, write the name of the compound having deep blue colours?
- The concentrated nitric acid produces yellow stains and blisters on skin. Give the reason.
- Write chemical equation when nitric acid reacts with sodium bicarbonate.
Section - 2
-
Q3.Give two reactions to show that nitric acid is:
- An acid
- An oxidizing agent
-
Q4.How will you show that nitric acid contains:
- Hydrogen?
- Nitrogen?
- Oxygen?
-
Q5.Give reasons for the following
- In the laboratory preparation of nitric acid, it can be obtained below 200°C or above 200°C, but the lower temperature is preferred.
- Commercial nitric acid is yellowish brown in color.
- Q6. Why is quartz in absorption tower packed in layers ?
- Q7. Write reactions involved in brown ring test used for detection of nitrate.
- Q8. “All apparatus are made of glass which are used in laboratory preparation of HNO 3 “. Why ?
- Q9. Name a nitrate which on heating gives oxygen as the only gaseous product. >
-
Q10. Complete the following equations-
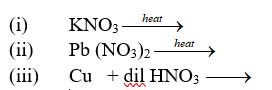
-
Q11. The manufacturing of nitric acid and sulphuric acid involves the use of a catalyst at some stage.
- Name the catalyst used in the manufacture
- Write the balanced equation for the reaction which involves the catalyst during the manufacture of nitric acid from ammonia.
- Q12. Nitric acid is now-a-days manufactured by the catalytic oxidation of ammonia. Describe briefly (in words or equations) the steps involved
-
Q13. Give correctly balanced equation for the laboratory preparation of nitric acid.
- What happens when some nitric acid falls on the hand?
- Atmospheric nitrogen undergoes a series of changed, represented in the diagram by lettered arrows into various compounds, named in the diagram.
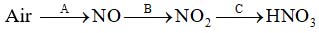 Give the equation for
Give the equation for
- Reaction A
- Reaction B
- Reaction C
- Q14. Explain, why neutral and colourless nitric oxide form acidic brown fumes on coming in contact with air.







