
Modern Concepts of Acids and Bases
Acid Base And Salt of Class 10
Following are the important modern concept of acids and bases:
Arrhenius Concept - The Water Ion System
According to this concept, an acid is any hydrogen containing compound which gives H + ions in aqueous solution and a base which gives OH – ions in aqueous solution. The HCl is an acid and NaOH is a base and the neutralisation process can be represented by a reaction involving the combination of H + and OH – ions to form H 2 O.
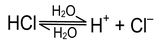
NaOH
 Na
+
+ OH
–
Na
+
+ OH
–
H + + OH – ⎯⎯→ H2O
Utility
- Since the reaction representing neutralisation process involves the combination of H + and OH – ions, the approximately constant molar heat of neutralisation would be expected. Thus the constant heat of neutralisation of a strong acid by a strong base is readily understandable in terms of this concept.
- This concept has offered a means of correlating catalytic behaviour with the concentration of the H + ion.
Limitations
- According to this concept, HCl is regarded as an acid only when dissolved in H2O and not in some other solvent such as C6H6 or when it exists in the gaseous form.
- The neutralisation process is limited to those reactions which can occur in aqueous solution only, although reactions involving salt formation do occur in many other solvents and even in the absence of solvents.
- It cannot explain the acidic character of certain salts such as AlCl3 in aqueous solution.

Bronsted - Lowry Theory - The Proton - donor - Acceptor System
This theory was given by Bronsted, a Danish chemist and Lowry, an English chemist independently in 1923, According to it an acid is a substance, molecule or ion which has a tendency to release the proton (protogenic) and similarly a base has a tendency to accept the proton (protophilic).
e. g.
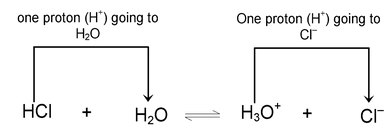
HCl + H 2 O --- H 3 O + + Cl -
In this reaction, HCl acts as an acid because it donates a proton to the water molecule. Water, on the other hand, behaves as a base by accepting a proton.
Bronsted and Lowry theory is also known as proton donor and proton acceptor theory.
Conjugate Acid - Base Pairs
Consider a reaction
Acid1 Base 2 Acid 2 Base1
H
2
O + NH
3
 H
3
O
+
OH
–
H
3
O
+
OH
–
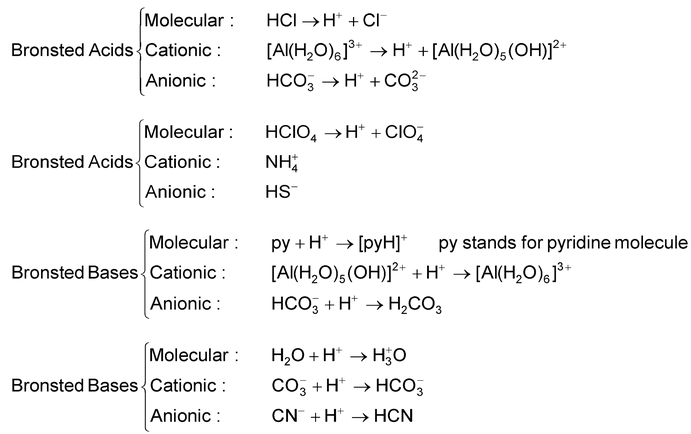
In this reaction HCl donates a proton to H 2 O and is, therefore an acid. Water, on the other hand, accepts a proton from HCl, and is, therefore, a base. In the reverse reaction which at equilibrium proceeds at the same rate as the forward reaction, the H 3 O + ions donates a proton to Cl – ion, hence H 3 O + , ion is an acid. Cl – ion, because it accepts a proton from H 3 O + ion, is a base. Acid base pairs such as.

The members of which can be formed from each other mutually by the gain or loss of proton are called conjugate acid - base pairs.
If in the above reaction, the acid HCl is labelled Acid1 and its conjugate base viz. Cl – as Base1 and further, if H 2 O is designated Base2 and its conjugate acid viz. H 3 O + as Acid 2, the equilibrium can be represented by a general equation.
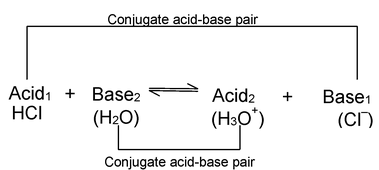
This is the fundamental equation representing the relationship between an acid and a base on the basis of Bronsted concept. Thus on the basis of this concept Acid1 and Base1 form one conjugate acid-base pair and Acid 2 and Base 2 form another conjugate acid-base pair.
Two important axioms of the Bronsted concept and position of equilibrium in acid-base reactions:
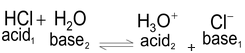
In the equilibrium mixture two acid HCl and H 3 O + ion are competing to donate protons to a base. Since HCl wins, it is the stronger acid. Similarly two bases, H 2 O and Cl – ion, are competing to accept protons. Here H2O is the stronger base. It will be seen that the stronger acid, HCl, has the weaker conjugate base Cl – ion and the stronger base, H 2 O, has weaker conjugate acid, H 3 O + ion. The stronger acid and weaker base form one conjugate acid - base pair and weaker acid and stronger base form another conjugate acid base pair. It is quite evident that HClO 4 is the strongest acid; its conjugate base ClO - 4 ion, is consequently the weakest base. CH 4 and H 2 are the weakest acids; their conjugate bases, CH - 3 ion and H – ion respectively, are consequently the strongest bases.
As a stronger acid, HCl is highly ionised even in concentrated aqueous solution. At equilibrium, the above reaction proceeds to the right, with most of HCl ionised to form H 3 O + and Cl – ions. This fact can be illustrated by using arrows of unequal length to designate the forward and reverse reactions respectively. Thus.
Stronger acid + Stronger Base
 Weaker acid + Weaker Base
Weaker acid + Weaker Base
HCl + H
2
O
 H
3
O
+
+ Cl
–
………..(1)
H
3
O
+
+ Cl
–
………..(1)
The longer arrow indicates that the position of equilibrium lies to the right.
In the ionisation of CH 3 COOH in H 2 O, equilibrium is reached when the reaction has proceeded to the right only to slight extent, with only a small fraction of the CH 3 COOH present in the form of ions.
Weaker acid + Weaker base
 Stronger acid + Stronger base
Stronger acid + Stronger base
CH
3
COOH + H
2
O
 H
3
O
+
+ CH
3
COO
–
……….. (2)
H
3
O
+
+ CH
3
COO
–
……….. (2)
Here the longer arrow indicates that the position of equilibrium lies to the left.
Evidently H 3 O + ion in equilibrium (2) is a stronger acid and CH 3 COO – ion is a stronger base. It is also evident that the stronger acid H 3 O + ion has the weaker conjugate base, H 2 O and the stronger base, CH 3 COO – has the weaker conjugate acid, CH 3 COOH. We thus see that all the proton transfer reactions (i.e., protolysis reactions) run downhill to form predominantly the weaker acid and the weaker base.
Also Check
Relative Strengths of Acids and Bases
According to Bronsted concept, a stronger acid has a stronger tendency to donate a proton and a strong base has a strong tendency to accept a proton. At least two general methods are generally used for the comparison of relative acidity of given acids.
- The first of these consists of making a comparison of proton-donating tendencies of different acids towards the same base. For moderately strong acids, H 2 O is generally used as the base. Suppose we have to compare the acidic strengths of CH 3 COOH and HCN. Experimentally it has been observed that the ionisation or acidity constant, Ka for CH 3 COOH and HCN at 25°C is 1.8 × 10 –5 and 4.0 × 10 –10 respectively.
CH
3
COOH + H
2
O
 H
3
O
+
+ CH
3
COO
–
(Ka = 1.8 × 10
–5
)
H
3
O
+
+ CH
3
COO
–
(Ka = 1.8 × 10
–5
)
HCN + H
2
O
 H
3
O
+
+ CN
–
(Ka = 4.0 × 10
–10
)
H
3
O
+
+ CN
–
(Ka = 4.0 × 10
–10
)
CH 3 COOH is, therefore, a stronger acid than HCN and CN – ion is a stronger base than CH 3 COO – ion.
- The second method is the competitive protolysis method. In this method one acid is added to the conjugate base of another and the equilibrium concentration are determined experimentally. For example, when NaOC 2 H 5 is added to H 2 O, it is experimentally seen that OC 2 H - 5 ion, which is the conjugate base of C 2 H 5 OH reacts fairly completely with H 2 O to form C 2 H 5 OH and OH – ion.
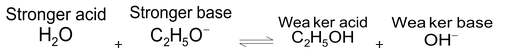
Ethoxide ion, C 2 H 5 O – is, therefore, a stronger base than OH – and H 2 O is a stronger acid than C 2 H 5 OH. Similarly when HS – is added to NH 3 , it has been found experimentally that NH 4 + and S 2– ions are present in the reaction mixture. This shows that NH 3 is a stronger base in comparison to HS – .

Lewis theory :
The theory was given by G.N. Lewis in 1938. According to it, an acid is a species which can accept a pair of electrons, while the base is one which can donate a pair of electrons.
It is also known as electron pair donor and electron pair acceptor theory.
e.g.
(i) FeCl 3 and AICI 3 are Lewis acids, because the central atoms have only six electrons after sharing and need two more electrons.
(ii) NH 3 is a Lewis base as it has a pair of electrons which can be easily donated.
Lewis acids :- CH 3 + , H + , BF 3 , AICI 3 , FeCl 3 etc.
Lewis base :- NH 3 , H 2 O, R-O-R, R - OH, CN - , OH - etc.
Characteristics of species which can act as Lewis acids:
(A)Molecules in which the central atom has incomplete octet: Lewis acids are electron deficient molecules such as BF3, AICl3, GaCl3 etc.
H 3 N : + AICI 3 → [H 2 N → AICl 3 ,]
Molecules in which the central atom has empty d-orbitals : The central atom of the halides such as TiCI4, SnCl4, PCI3, PF5, SF4, TeCl4. etc., have vacant d-orbitals. These can, therefore, accept an electron pair and act as Lewis acids.
(C) Simple cations : All cations are expected to act as Lewis acid, since they are electron deficient in nature.
Ag + + 2NH 3 → [H 3 N→Ag ←NN 3 ] +
Fe 2+ + 6CN - → [Fe(CN) 6 ] 4 -
(D) Molecules having a multiple bond between atoms of dissimilar electronegativity : Typical examples of molecules belonging to this class of Lewis acids are CO 2 , SO 2 and SO 3 .
Characteristics of species which can act as Lewis bases :
(A) Neutral species having at least one lone pair of electrons: For example, ammonia amines, alcohols etc, act as Lewis bases as they contain a pair of electrons.
(B) Negatively charged species or simple anions: For example chloride (CI - ), cyanide (CN - ), hydroxide (OH - ) ions etc. act as Lewis bases.
(C)Multiple bonded compounds : The compounds such as CO, NO, ethylene, acetylene etc. can act as Lewis bases.
Utility of Lewis concept
- This concept also includes those reactions in which no protons are involved.
- Lewis concept is more general than the Bronsted - Lowry concept (i.e. protonic concept) in that acid-base behaviour is not dependent on the presence of one particular element or on the presence or absence of a solvent.
- It explains the long accepted basic properties of metallic oxides and acidic properties of non- metallic oxides
- This theory also includes many reactions such as gas phase, high temperature and non- solvent reaction as neutralisation process.
- The Lewis approach is, however, of great value in case where the protonic concept is inapplicable, for example, in reaction between acidic and basic oxides in the fused state.
Limitations
- Since the strength of Lewis acids and bases is found to depend on the type of reaction, it is not possible to arrange them in any order of their relative strength. Thus, for example, experiments show that fluoride complex of Be 2+ ions is more stable than that of Cu 2+ ion, indicating that Be 2+ ion is more acidic than Cu 2+ ion. On the other hand amine complex of Cu 2+ is more stable than that of Be 2+ ion indicating that Cu 2+ is more acidic than Be 2+ ion.
- According to the phenomenological criteria, an acid-base reaction should be a rapid reaction. There are, however, many Lewis acid-base reactions which are slow.










