
Chemical Properties Of Bases
Acid Base And Salt of Class 10
Chemical Properties of Bases
- The bases change the color of the litmus from red to blue.
- They are bitter in taste.
- Bases lose their base when mixed with acids.
- The bases react with the acids and form salt and water. This complete process is known Neutralization Reaction (Read).
- They can generate electricity as well.
- The bases feel slippery or soapy.
- Some bases are good electrical conductors. Bases such as sodium hydroxide, potassium hydroxide, etc. are used as electrolytes.
- Alkalis are the bases that produce hydroxyl ions (OH - ) when mixed with water.
- Strong alkalis naturally rust naturally and some alkalis are mildly corrosive.
- The pH value of the bases ranges from 8-14.
- The Alkalis and ammonium salts produce ammonia.
- Hydrogen gas changes when metals react at the base.
- The bases are divided on the basis of strength, concentration and acidity.
- Different types of acids are strong acid, weak base acid, concentrated base, dilute base, monoacidic base, diacidic base and triacidic base.
REACTION OF BASES WITH METALS:
Metals like zinc, tin and aluminum react with strong alkalies like NaOH (caustic soda), KOH (caustic potash) to evolve hydrogen gas.
Zn(s) + 2NaOH(aq) → Na 2 ZnO 2 (aq) + H 2 (g)
Sodium zincate
Sn(s) + 2NaOH(aq) → Na 2 SnO 2 (aq) + H 2 (g)
Sodium stannite
2AI(s) + 2NaOH + 2H 2 O → 2NaAIO 2 (aq) + 3H 2 (g)
Sodium meta aluminate
Experiment: Take 2-3 pieces of zinc granules in a test tube and add about 2-3 ml of conc. NaOH solution in to it and warm the contents.
Observation: There is evolution of H 2 gas which burns with a pop sound (on bringing a burning candle near the mouth of tube).
The reaction involved is:
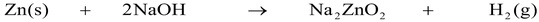
zinc sodium hydroxide sodium zincate hydrogen
(a metal) (conc.) (a salt) gas
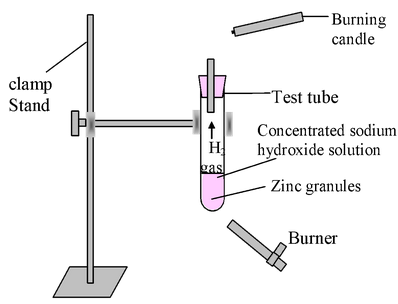
Figure-Study of the reaction of sodium hydroxide with Zn metal
All metals do not react with bases to form salts and hydrogen gas.
REACTION OF BASES WITH ACIDS (NEUTRALIZATION REACTION)
When a base reacts with an acid then salt and water are formed
i.e. Base + Acid → Salt + Water
This reaction is called neutralization reaction, because when base and acid react with each other, they neutralize each other’s effect (i.e. acid destroys the basic property of a base and a base destroys the acidic property of an acid)
(i)

sodium hydroxide hydrochloric acid sodium chloride water
(base) (acid) (salt)
(ii)

sodium hydroxide sulphuric acid sodium sulphate water
(base) (acid) (salt)
Conclusion: Reaction of a base with an acid is a neutralization of an acid by base
REACTION OF BASE WITH NON-METAL OXIDE:
Bases react with non-metal oxide to form salt and water
i.e. Non-metal oxide + Base → Salt + water
This reaction is similar to the neutralization reaction between acid and base to form salt and water. Thus, the reaction between bases and non-metal oxides is a kind of neutralization reaction and shows that non-metal oxides are acidic oxides.
⇒ Reaction of calcium hydroxide (lime water) with carbon dioxide.
Calcium hydroxide (lime water) is a base and carbon dioxide (CO 2) is a non-metal oxide, so when they react with each other, salt and water are produced according to the reaction:

calcium hydroxide carbondioxide calcium water
(lime water) (non-metal oxide) carbonate
(base) (salt)
2NaOH(aq) + CO 2 (g) → Na 3 CO 3 (aq) + H 2 O
Ca(OH) 2 (s) + SO 2 (g) → CaSO 3 (aq) + H 2 O
Conclusion: Reactions of bases with non-metal oxides are neutralization reactions which show the acidic nature of non-metal oxide.

Related Topics





