Atoms
Atom and Molecule of Class 9
Houses are made up of bricks. Similarly all matter whether an element, or a compound or a mixture is made up of the smallest indivisible particles called atoms. In other word atoms are the building blocks of all matter. In chemistry atom is defined as the smallest particle of an element that can take part in a chemical reaction and which may or may not be capable of free existence. Atoms of most of the elements are very reactive and do not exist in the free state, instead, they exist in combination with the atoms of the same element or another element. For example, H2, N2, O2, HCl, NH3, etc.
Atoms are so small that we cannot see them under the most powerful optical microscope.
Hydrogen atom is the smallest atom of all, having an atomic radius 0.037 nm.
Atomic radii of some common elements
|
S. No. |
Element |
Atomic radius |
|
1. 2. 3. 4. 5. 6. |
Hydrogen Carbon Nitrogen Oxygen Chloride Sulphate |
0.037 nm 0.077 nm 0.074 nm 0.073 nm 0.099 nm 0.104 nm |
Atoms are so small that they cannot be seen even under the most powerful optical microscope. However it is only recently that a highly sophisticated microscope known as scanning tunneling microscope (STM), has magnified image of surface of elements showing atoms.
|
Relative size |
|
|
Radii (in meter) |
Example |
|
10 –10 |
Molecule of water |
|
10 –9 |
Atom of hydrogen |
|
10 –8 |
Molecule of haemoglobin |
|
10 –4 |
Grain of sand |
|
10 –3 |
Ant |
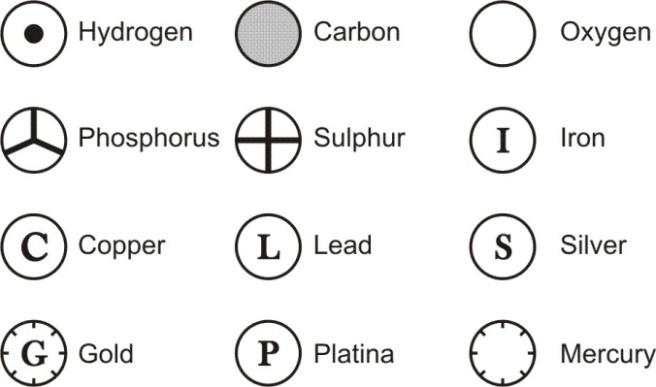
DALTON’S SYMBOLS OF ELEMENT:
|
Dalton was the first scientist to suggest the symbols for elements in a very specific way. Dalton’s symbol for an element represent the “element’ as well as one atom of that element. Thus, we can say that the symbol used by him also represent the quantity of the element. A few of these symbols as proposed by Dalton are as follows : Dalton’s symbols were difficult to draw and inconvenient to use so they could not become popular. |
|
BERZELIUS SUGGESTION FOR SYMBOLS OF ELEMENTS:
J.J. Berzelius, a Swedish chemist, suggested a more scientific method for representing an element. He suggested that the first one or two letter of the name of an element can be used as its symbols. This idea led to the development of modern symbols of elements.
MODERN SYMBOLS OF ELEMENTS:
In the beginning, the names of elements were derived from the name of the place where they were first found, discovered or after the name of the scientist who discovered it. For example the name copper was taken from Cyprus, Rutherfordium after Rutherford etc. Some names were taken from specific colours. For example gold was taken from the English word meaning yellow. However as more and more elements were discovered, an international committee was set up called ‘International Union of Pure and Applied Chemistry’ (IUPAC) which approved the names of different elements.
Though the names of most of the elements have been taken from English there are some elements which have been taken from Latin, German or Greek. In all cases the symbol of an element is the “first letter” or “the first letter and another letter” of the English or Latin name of the element. For example:
The symbol of Hydrogen is H
The symbol of Oxygen is O
So, in the case of hydrogen and oxygen the first letter of their English names are taken as their symbols.
It should be noted that in a “two letter” symbol, the first letter is the capital letter but the second letter is the small letter. The necessity of adding another letter arises only in case of elements whose names start with the same letter. For example the name of the elements viz. carbon, chlorine calcium and copper starts with the common letter C.
Hence,
Carbon is represented by the symbol C
Chlorine is represented by the symbol Cl
Calcium is represented by the symbol Ca
Copper is represented by the symbol Cu
Symbols of elements derived from English names
|
English name of the element |
Symbol |
English name of the element |
Symbol |
|
1. Argon |
Ar |
15. Iodine |
I |
|
2. Arsenic |
As |
16. Lithium |
Li |
|
3. Aluminium |
Al |
17. Magnesium |
Mg |
|
4. Boron |
B |
18. Manganese |
Mn |
|
5. Barium |
Ba |
19. Nitrogen |
N |
|
6. Bromine |
Br |
20. Neon |
Ne |
|
7. Carbon |
C |
21. Nickel |
Ni |
|
8. Calcium |
Ca |
22. Oxygen |
O |
|
9. Chlorine |
Cl |
23. Phosphorus |
P |
|
10. Chromium |
Cr |
24. Platinum |
Pt |
|
11. Cobalt |
Co |
25. Sulphur |
S |
|
12. Fluorine |
F |
26. Uranium |
U |
|
13. Hydrogen |
H |
27. Zinc |
Zn |
|
14. Helium |
He |
Symbols derived from Latin names
|
English name of the element |
Latin name of the element |
Symbol |
|
1. Antimony |
Stibium |
Sb |
|
2. Copper |
Cuprum |
Cu |
|
3. Gold |
Aurum |
Au |
|
4. Iron |
Ferrum |
Fe |
|
5. Lead |
Plumbum |
Pb |
|
6. Mercury |
Hydrargyrum |
Hg |
|
7. Potassium |
Kalium |
K |
|
8. Silver |
Argentum |
Ag |
|
9. Sodium |
Natrium |
Na |
|
10. Tin |
Stannum |
Sn |
ATOMIC MASS OF AN ELEMENT:
Actual masses of the atoms of the elements are very, very small. For example, one atoms of hydrogen (H) has mass of 1.673 × 10 -24 gram. To avoid the inconvenience in using such small and complicated figures in our calculation, it was necessary to define atomic mass in such as way that we get simple figures for them. Carbon -12 atom is that atom of carbon which has 6 protons and 6 neutrons in its nucleus, so that its mass number is 12.
Carbon -12 atom has been assigned an atomic mass of exactly 12 atomic mass units, written as 12 u.
Definition of atomic mass : Atomic mass express as to how many time an atom of a substance is heavier than
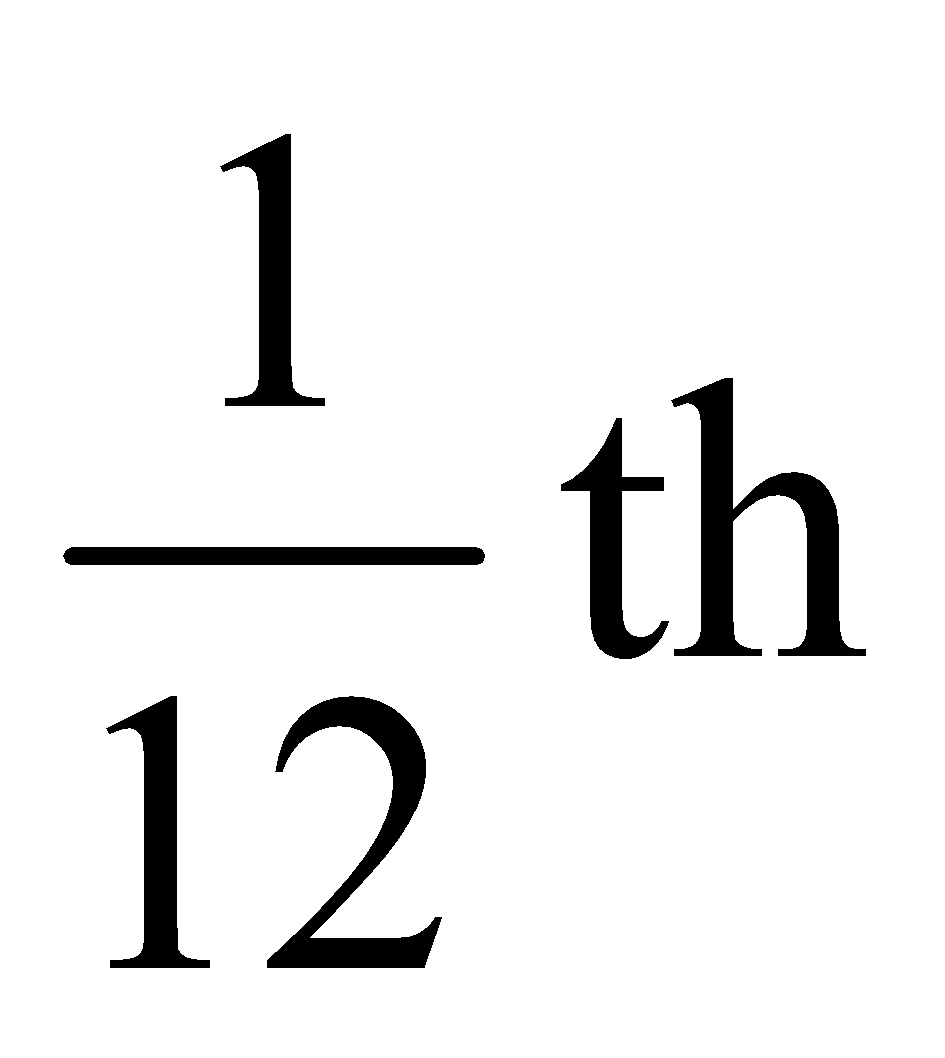 mass of the carbon - 12 atom.
mass of the carbon - 12 atom.
Atomic Mass Unit (u) = One – twelfth the mass of a Carbon – 12 atom or 1u = 1.6605 × 10 -24 g.
One atomic mass unit (1u) is defined as exactly one-twelfth the mass of an atom of Carbon-12. The atomic mass of an element is the relative mass of its atom as compared with the mass of a Carbon-12 atom taken as 12 units.
Atomic Mass Unit (u) = One - twelfth the mass of a Carbon - 12 atom or 1u = 1.6605 × 10 -24 g.
|
Element |
Symbol |
Atomic mass |
Element |
Symbol |
Atomic mass |
|
1. Hydrogen |
H |
1 |
14. Sulphur |
S |
32 |
|
2. Helium |
He |
4 |
15. Chlorine |
Cl |
35.5 |
|
3. Lithium |
Li |
7 |
16. Argon |
Ar |
40 |
|
4. Boron |
B |
11 |
17. Potassium |
K |
39 |
|
5. Carbon |
C |
12 |
18. Calcium |
Ca |
40 |
|
6. Nitrogen |
N |
14 |
19. Iron |
Fe |
56 |
|
7. Oxygen |
O |
16 |
20. Copper |
Cu |
63.5 |
|
8. Fluorine |
F |
19 |
21. Zinc |
Zn |
65 |
|
9. Neon |
Ne |
20 |
22. Silver |
Ag |
108 |
|
10. Sodium |
Na |
23 |
23. Platinum |
Pt |
195 |
|
11. Magnesium |
Mg |
24 |
24. Gold |
Au |
197 |
|
12. Aluminium |
Al |
27 |
25. Lead |
Pb |
207 |
|
13. Phosphorus |
P |
31 |
26. Uranium |
U |
238 |
HOW DO ATOMS EXIST?
Atoms of most elements are not able to exist independently. Only the atoms of a few elements exist in free state.
Atoms usually exist in two ways :
- In the form of molecules
- In the form of ions
When they form molecules or ions they become stable. Molecules and ions aggregate in large numbers to form matter which we see around us.
Though we cannot see individual atoms, molecules or ions but we can see the matter. For example, we cannot see the Na + and Cl − ions but we can see the sodium chloride compound (common salt).