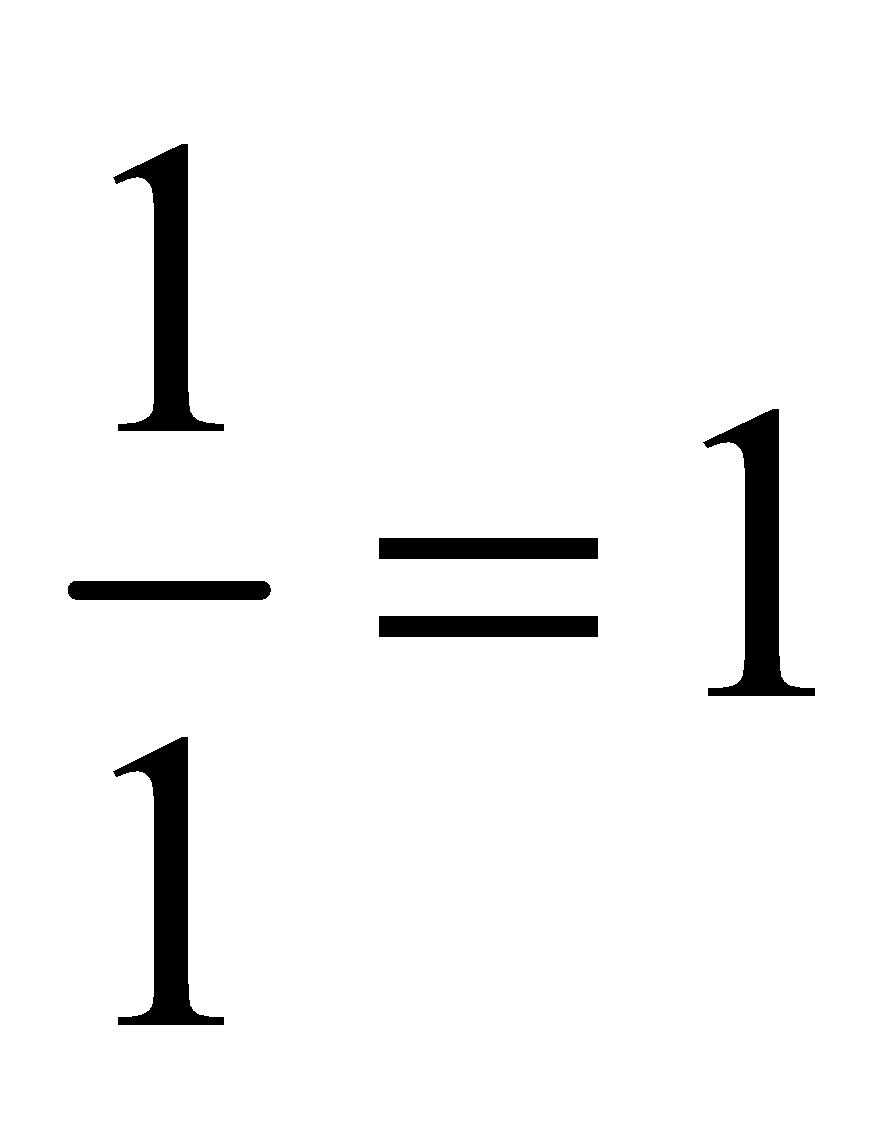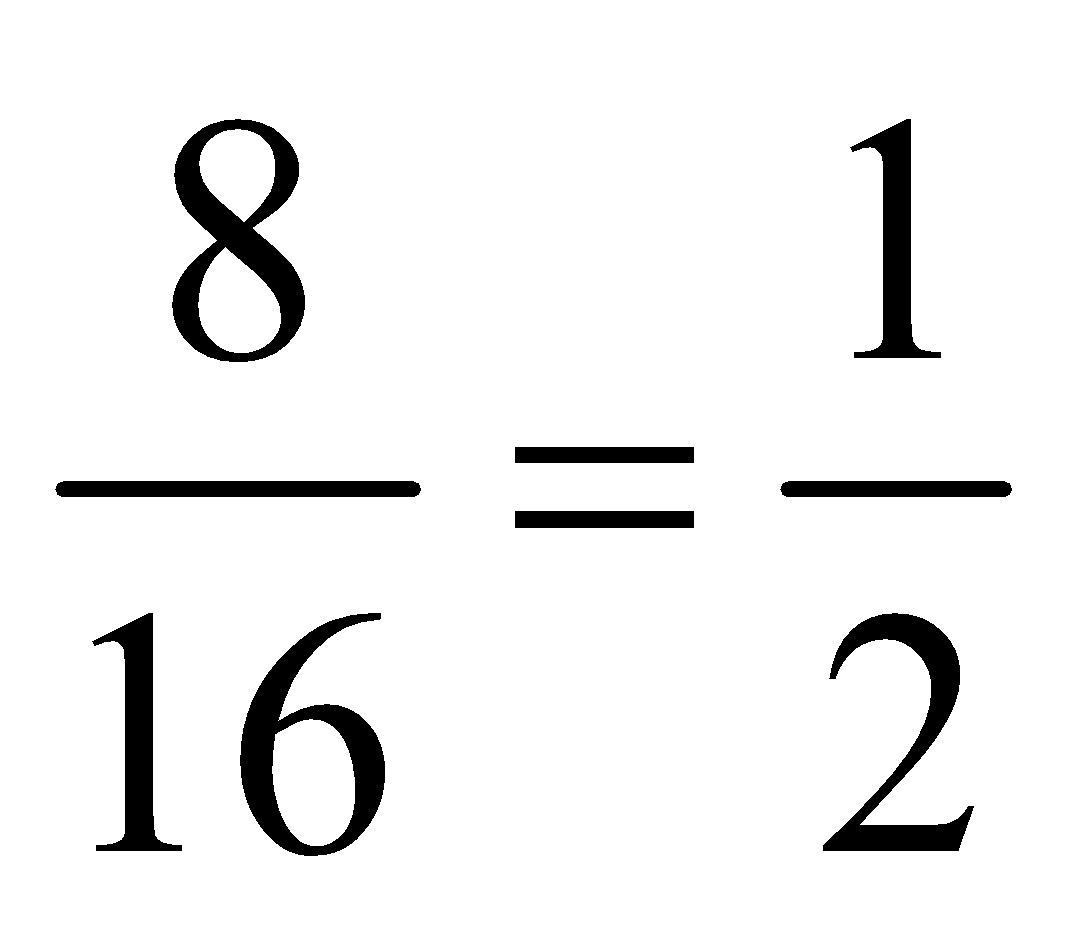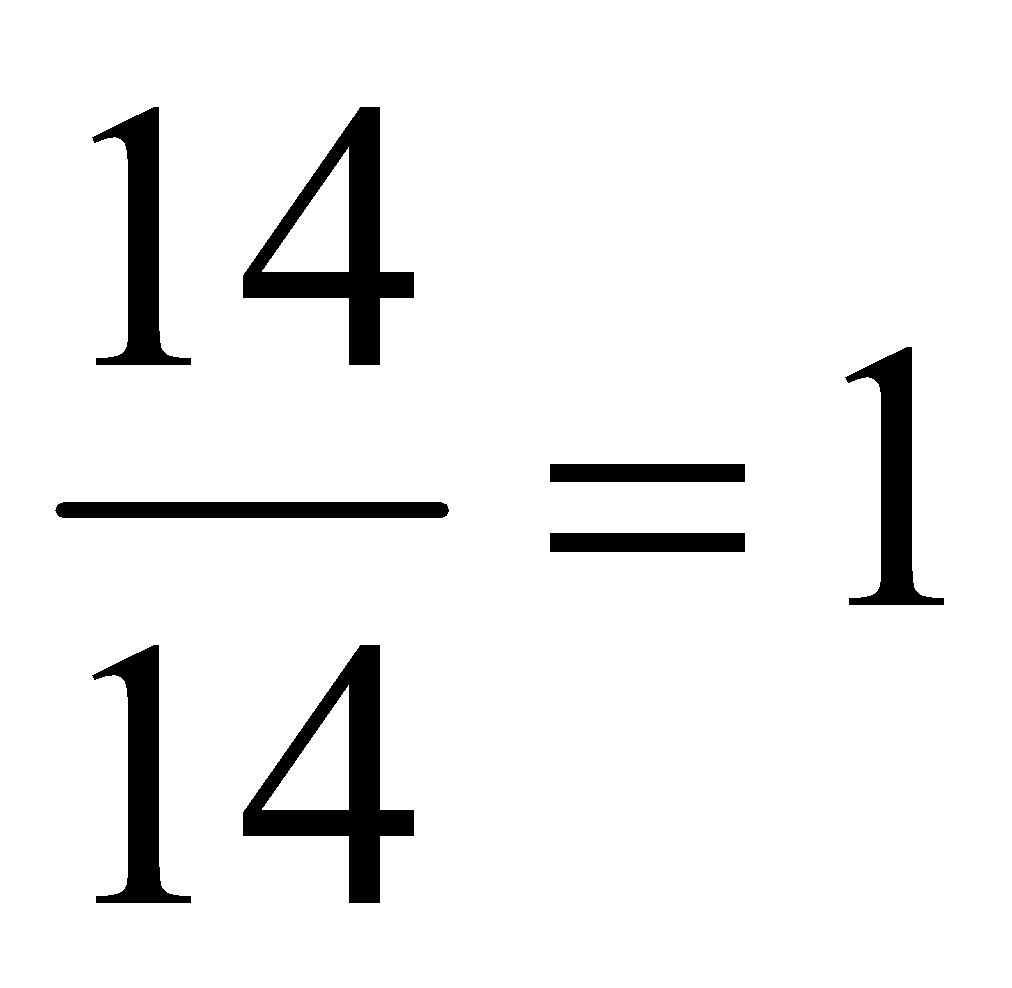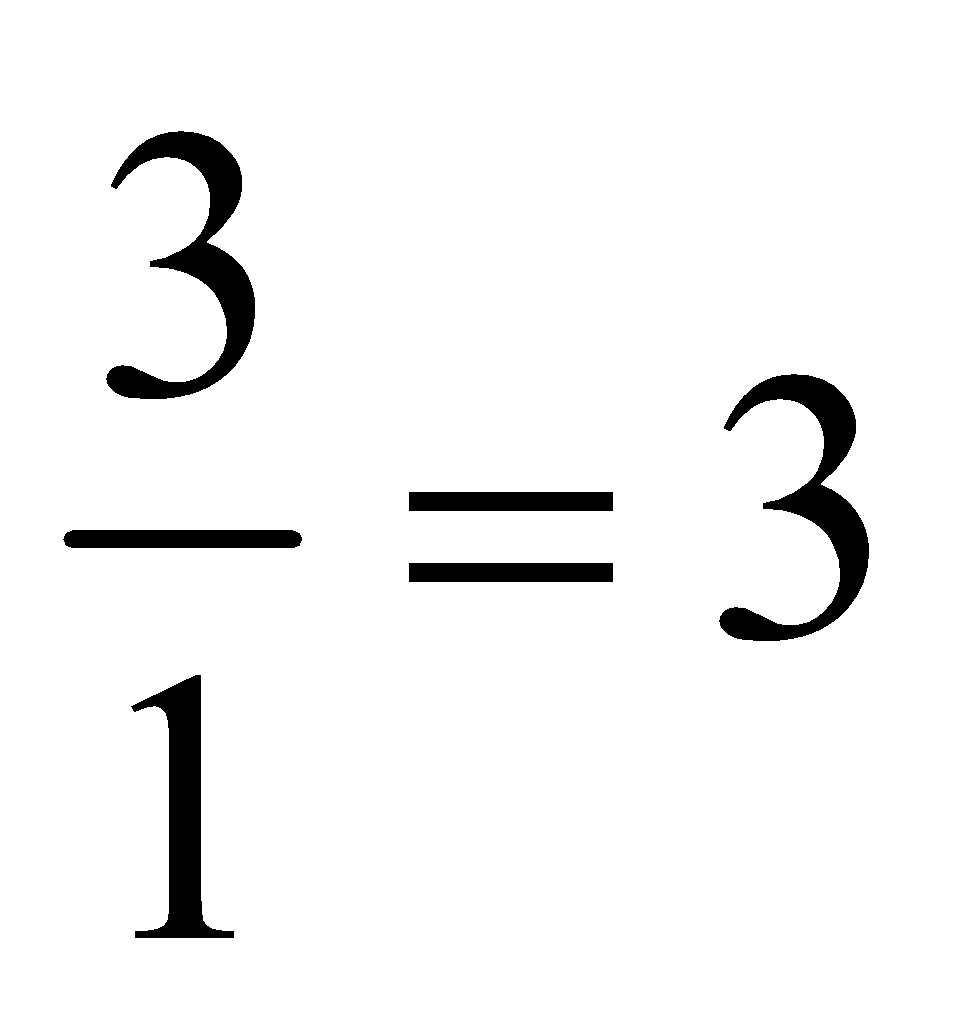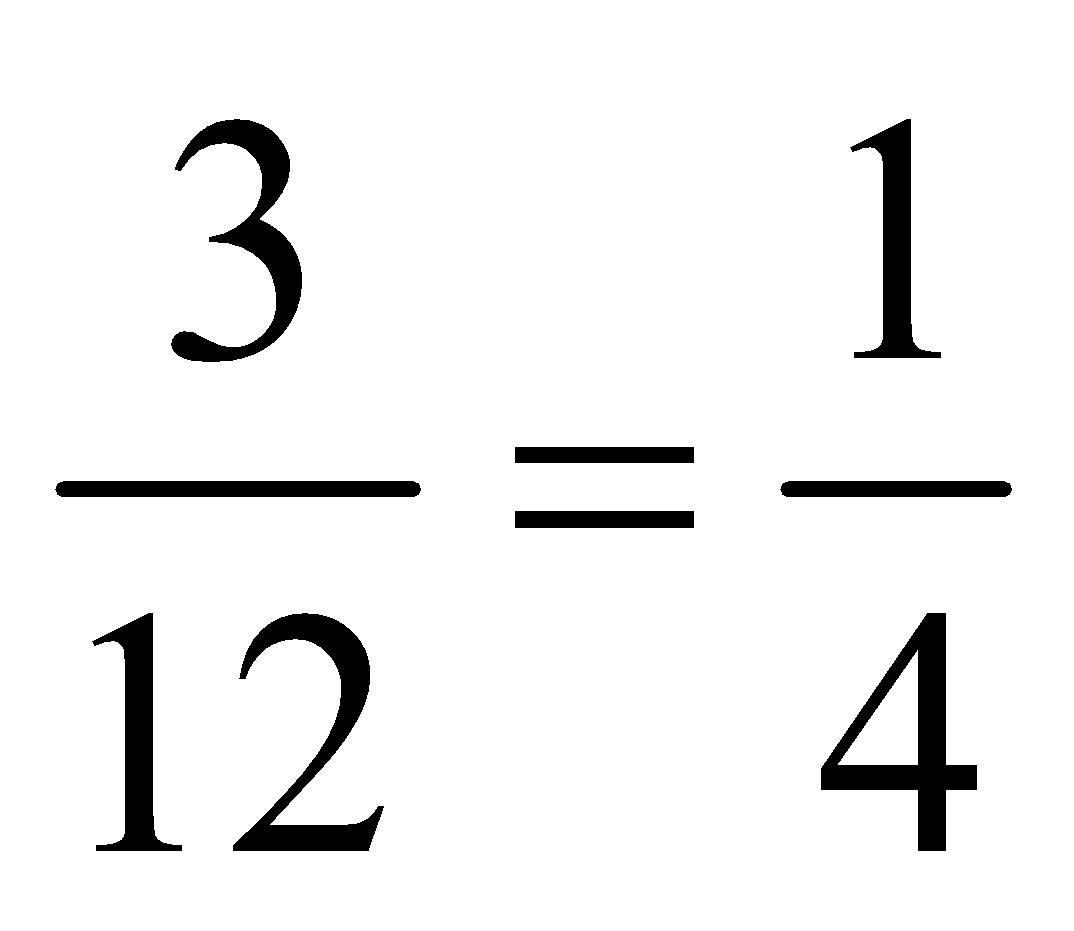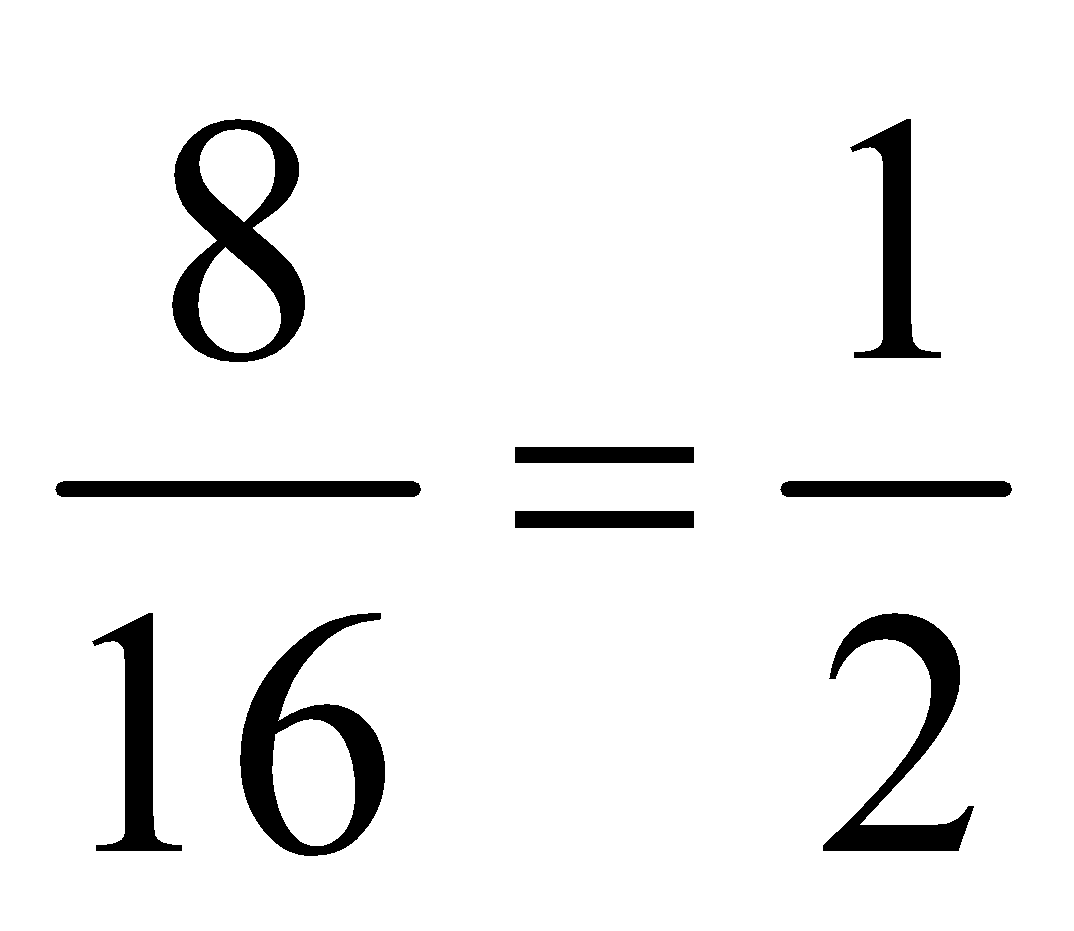what is Molecule
Atom and Molecule of Class 9
A molecule is an electrically neutral group of two (or more) atoms chemically bonded together by means of attractive forces.
OR
A molecule is the smallest particle of a substance (element or compound) which has the properties of that substance and can exist in the free state. Molecules can be formed either by the combination of atoms of the “same element” or of “different elements”/
There are two types of molecules : molecules of elements and molecules of compounds.
MOLECULE OF AN ELEMENT:
The molecules of an element are constituted by the same type of atoms.
OR
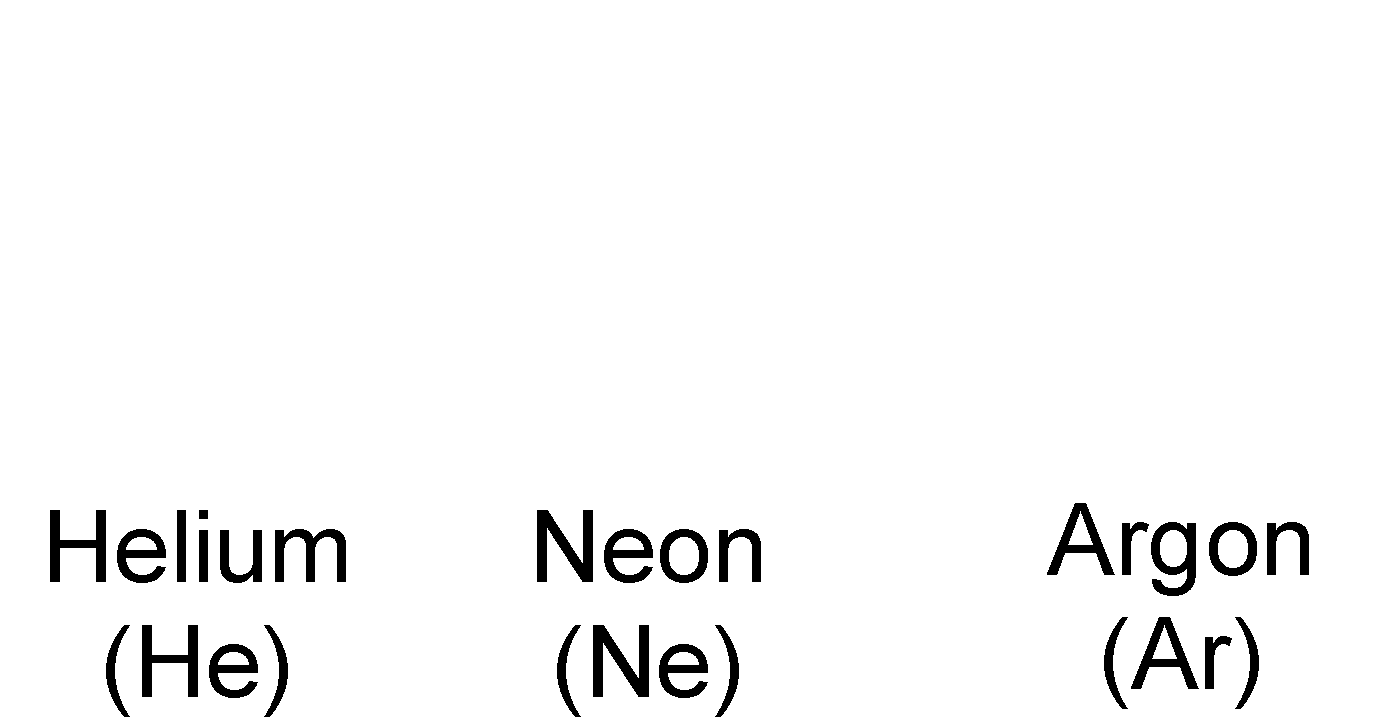
The molecules of an element contain two (or more) similar atoms chemically combined together. Molecules of many elements such as Argon (Ar), Helium (He), etc. are made up of only one atom of that element. But this is not the case with most of the elements. Depending upon whether the molecule contains one, two, three or four atoms they are called monoatomic, diatomic triatomic, tetra atomic or polyatomic. A few examples of molecules of different types are as follows:
Monoatomic molecules : Noble gases like Helium, Neon, etc. exist as single atoms i.e. He, Ne etc. Hence they are called monoatomic.
Diatomic molecules : Molecules of hydrogen, oxygen, nitrogen contain two atoms of each element respectively and are represented by H2, O2, N2 etc.
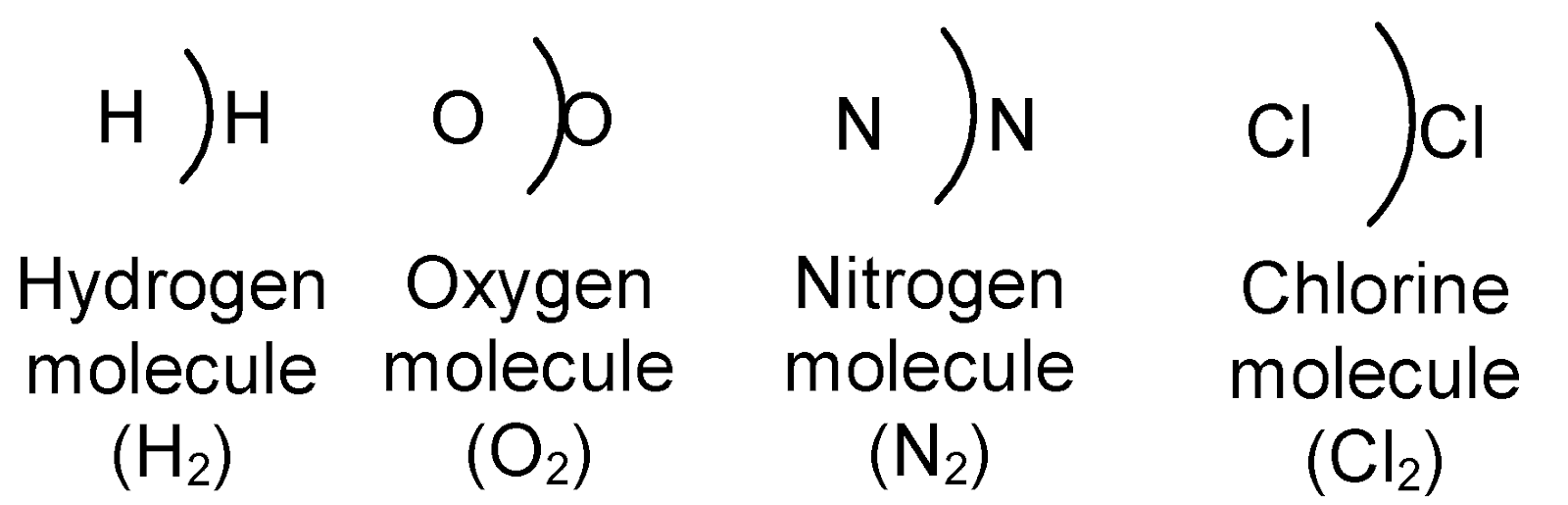
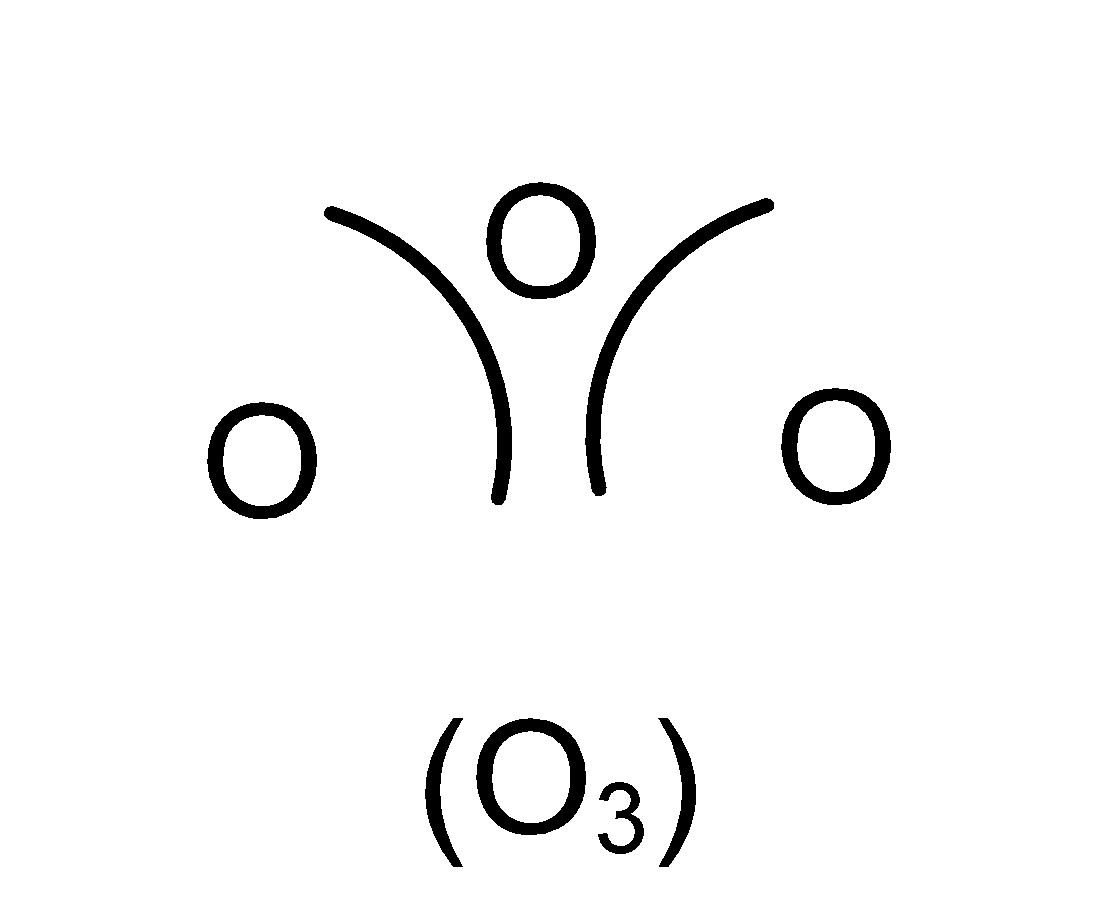
Triatomic molecules : Molecules containing 3 atoms are called triatomic molecules. For example, ozone contains 3 atoms of oxygen combined together.
Tetratomic molecules : Molecules containing 4 atoms of an element are called tetratomic molecules. Most common example is that of phosphorus represented by P4.
Polyatomic molecules : Molecules containing more than four atoms of particular element are called polyatomic molecules. For example, a molecule of sulphur contains 8 atoms of sulphur and is represented by S8.
Atomicity: The number of atoms present in one molecule of an element or compound is called it atomicity. For example, the atomicity of noble gases is 1, that of hydrogen, nitrogen, oxygen etc. is 2 each and of ozone is 3. Thus, noble gases, hydrogen and ozone are respectively monatomic, diatomic, and triatomic molecules.
A compound which consists of molecules and not ions, is called a molecular compound.
Atomicity of some elements
The atomicity of some elements can be shown in the following tables:
|
Atomicity of some molecules |
||
|
Type of Element |
Name |
Atomicity |
|
Non-metal |
Argon Helium Oxygen Hydrogen Nitrogen Chlorine Phosphorus Sulphur |
Monoatomic Monoatomic Diatomic Diatomic Diatomic Diatomic Tetra-atomic Poly-atomic |
MOLECULES OF COMPOUNDS:
The molecules of a compound consist of two or more atoms of different elements combined together in a definite proportion by mass to form a compound that can exist freely.
For example, carbon dioxide contains one atom of carbon and two atoms of oxygen combined together in a fixed ratio of 3 : 8 by mass.
|
Molecules of some compounds |
||
|
Compound |
Combining Elements |
Ratio by Mass |
|
Water Ammonia Carbon dioxide |
Hydrogen, Oxygen Nitrogen, Hydrogen Carbon, Oxygen |
1:8 14:3 3:8 |
In the above table we have given the ratio by mass of atoms present in one molecule of that particular compound. The atomic masses of different elements are H = 1.0u, O = 16.0u, N = 14.0u, C = 12.0u. By comparing the data we can find out the ratio by number of atoms of elements in the molecule of the particular compound as follows:
|
S. No. |
Compound |
Element |
Atomic mass(u) |
ratio by mass |
Mass ratio/ atomic mass |
simplest ratio |
|
1. |
H 2 O |
H |
1 |
1 |
|
2 |
|
O |
16 |
8 |
|
1 |
||
|
2. |
NH 3 |
N |
14 |
14 |
|
1 |
|
H |
1 |
3 |
|
3 |
||
|
3. |
CO 2 |
C |
12 |
3 |
|
1 |
|
O |
16 |
8 |
|
2 |
Thus ratio by number of atoms for water is H : O = 2:1, for ammonia is N:H = 1:3 and for carbon dioxide C:O = 1:2. Thus we can say that in a compound, the element are combined together in a simple whole number atomic ratio.