
Nomenclature And Classification Of Hydrocarbons
Carbon And Its Compound of Class 10
NOMENCLATURE AND CLASSIFICATION OF HYDROCARBONS
Nomenclature of Aliphatic Compounds
According to IUPAC system, the name of organic compound, in general consists of the following parts.
(I) Word root (II) Primary suffix
(III) Secondary suffix (IV)Prefix
(I) Word root
The word root represents the number of carbon atoms in the parent chain. For the chains upto four carbon atoms special word roots are used, but for the chains containing more than four carbon atoms, Greek numerals are used.
Word Roots or different lengths of Carbon Chains
|
Chain Length |
Word root |
Chain Length |
Word root |
|
C1 C2 C3 C4 C5 C6 C7 C8 |
Meth− Eth− Prop− But− Pent Hex− Hept− Oct− |
C9 C10 C11 C12 C13 C18 C20 C30 |
Non− Dec− Undec− Dodec− Tridec− Octadec− Icos− Tricont− |
Primary Suffix
Primary suffix is used to represent saturation or unsaturation in the carbon chain. While writing the name, primary suffix is added to the word root.
Some Primary Suffixes
Nature of Carbon Chain |
Primary Suffix |
|
Saturated Carbon Chain Unsaturated Carbon Chains one C = C bond two C = C bonds three C = C bonds one C ≡C bond two C ≡C bonds one C = C and one C≡C |
ane ene adiene atriene yne adiyne enyne |
Secondary Suffix
Secondary suffix is used to indicate the functional group in the organic compound. The terminal 'e' of the primary suffix is dropped if it is followed by a suffix beginning with 'a', 'i', 'o', 'u' or 'y'.
|
Class of organic compound |
General formula |
Suffix |
IUPAC name of the family (Word root + Primary suffix + Secondary suffix) |
|
Alcohols |
R—OH |
−OH |
Alkanol |
|
Thioalcohols |
R—SH |
−SH |
Alkanethiol |
|
Amines |
R—NH 2 |
−NH2 |
Alkanamine |
|
Aldehydes |
R—CHO |
−CHO |
Alkanal |
|
Ketones |
R—COR |
>CO |
Alkanone |
|
Carboxylic acids |
R—COOH |
−COOH |
Alkanoic acid |
|
Amides |
R—CONH 2 |
−CONH 2 |
Alkanamide |
|
Acid chlorides |
R—COCl |
−COCl |
Alkanoyl chloride |
|
Esters |
R—COOR |
−COOR |
Alkyl alkanoate |
|
Nitriles |
R—C≡N |
−C≡N |
Alkanenitrile |
Note:
- If the name of the secondary suffix begins with a consonant, then the terminal 'e' of the primary suffix is not dropped while adding secondary suffix to it.
- (The terminal 'e' of primary suffix is also retained if some numerical prefix like di, tri, etc. is used before the secondary suffix.
Prefix
Prefix is a part of the name which appears before the word root. Prefixes are used to represent the names of alkyl groups (branched chains) or some functional groups which are regarded as substituents.
Some hydrocarbon Groups along with their Prefixes
|
Alkane |
Alkyl group |
Abbreviation |
Prefix |
|
CH4 C2H6 C3H8 C3H8 |
CH 3 − CH 3 CH2− CH 3 CH 2 CH 2 − CH 3 —CH− | CH 3 CH 2 = CH− CH ≡ C − |
Me− Et− n−Pr− Iso−Pr− − − |
Methyl Ethyl n−Propyl isoproyl or (1−Methyl ethyl) ethenyl or vinyl ethynyl |
Some functional group which are always represented by prefixes
|
Functional group |
Prefix |
Family |
IUPAC name |
|
−NO 2 −OR −Cl −Br −I −F −NO |
Nitro alkoxy Chloro Bromo Iodo Fluoro Nitroso |
R−NO2 R−OR R−Cl R−Br R−I R−F R−NO |
Nitroalkane Alkoxyalkane Chloroalkane Bromoalkane Iodoalkane Fluoroalkane Nitrosoalkane |
Arrangement of Prefixes, Word root and Suffixes: The prefixes, word root and suffixes are arranged as follows while writing the IUPAC name.
Prefix(es) + word root + primary suffix + secondary suffix
Various Rules For Writing Names
In order to have better understanding of various rules of nomenclature, let us discuss it in different parts as
- Nomenclature of hydrocarbons.
- Nomenclature of compounds containing one functional group.
- Nomenclature of compounds with more than one similar functional groups.
- Nomenclature of compounds with more than one dissimilar functional groups.
Nomenclature of Hydrocarbons
Hydrocarbons are named according to the following general rules.
Rule 1: Longest Chain Rule
Select the longest continuous chain of carbon atoms as the parent chain. If some carbon−carbon multiple bond is present, the parent chain must contain the carbon atoms involved in it. The number of carbon atoms in the parent chain determines the word root. The carbon atoms which are not included in the parent chain are considered as alkyl substituents and determine the prefixes.
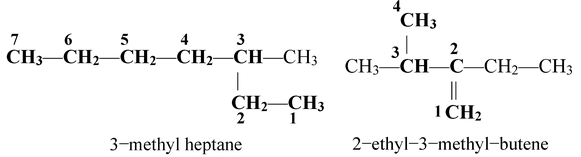
If two equally long chains are possible, the chain with maximum number of side chains is selected as parent chain. For example,
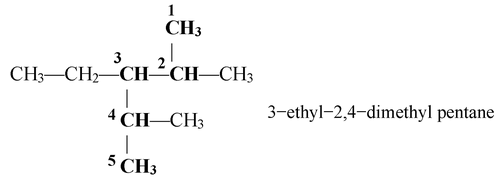
In some cases, the longest chain is ruled out at the expense of conjugated system. For example,
Rule 2: Lowest Number or Lowest Sum Rule
The selected parent chain is numbered using Arabic numerals and the positions of the alkyl groups are indicated by the number of the carbon atom to which the alkyl group is attached. The numbering is done in such a way that the substituted carbon atoms have the lowest possible numbers. When series of locants containing the same number of terms are compared by term, that series is lowest which contains the lowest number on the occasion of first difference. Some examples are given below:
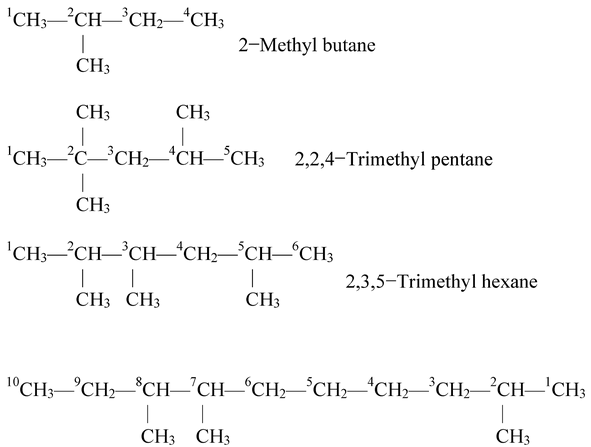
2,7,8−Trimethyldecane
2,7,8Trimethyldecane is the correct name of the above compound 3,4,9−Trimethydecane is wrong name through it has the lowest sum of numbers. The first name is correct because in the first case substituent gets lower locant value than the second one.
In case of unsaturated hydrocarbons, the carbon atoms involved in the multiple bond should get the lowest possible number, For example.
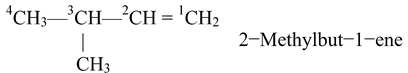
The name of the compound, in general is written in the following sequence:
(Position Of Substituents)−(Prefixes) (Word Root) (Primary Suffix)
The above sequence is illustrated in the following examples.
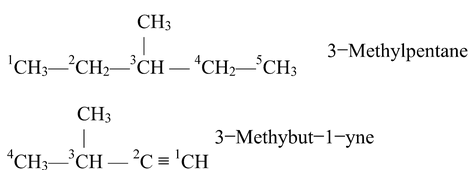
Rule 3: Use of Prefixes Di, Tri, etc.
If the compound contains more than one similar alkyl groups, their positions are indicated separately and an appropriate numerical prefix: di, tri, etc. is attached to the same of the substituent. The positions of the substituents are separated by commas. For example,
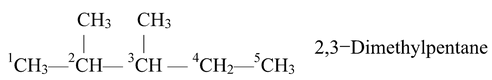
Rule 4. Alphabetical Arrangement of Prefixes
If there are different alkyl substituents present in the compound, their names are written in the alphabetical order. However, the numerical prefixes such as di, tri, etc are not considered for the alphabetical order. For example.
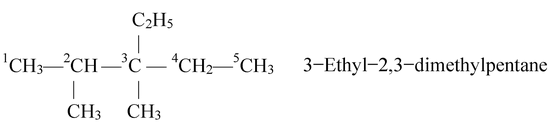
Naming different alkyl substituents at the equivalent positions. If two different alkyl groups are located at the equivalent positions, then numbering of the chain is done in such a way that the alkyl group which comes first in alphabetic order gets the lower position. For example, if ethyl and methyl groups are present in equivalent positions, then carbon bearing ethyl group should get the lower number as illustrated in the following example.
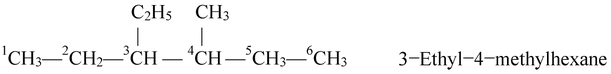
In the light of the above rules, let us write the IUPAC name of some alphatic hydrocarbons.
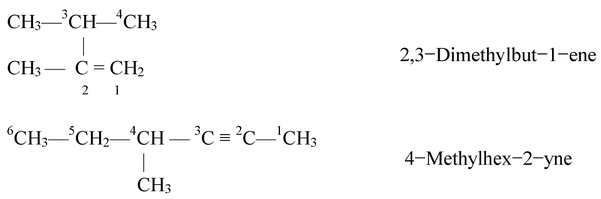
Rule 5: Naming the Complex Alkyl Substituents
If the alkyl substituent is further branched it is named as substituted alkyl group. For this purpose, the carbon atoms of the alkyl group are separately numbered in such a way, that the carbon atom directly attached to the parent chain is given number 1. the prefix/name of such a substituent is enclosed in brackets as illustrated in the following example.
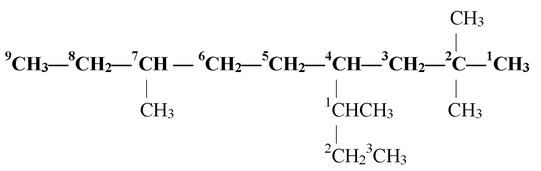
Here substituted propyl group is present at carbon number 4 of the parent chain. The propyl group has two methyl substituents as carbon number 1 of the alkyl group. Thus, the name of the compound is 2,2,7−Trimethyl− 4 − (1−methylpropyl) nonane
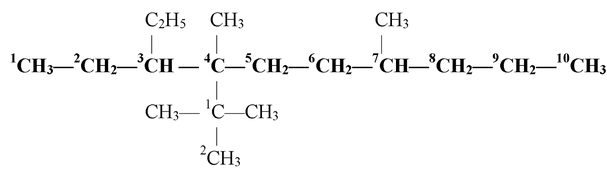
Here substituted ethyl group is present at carbon number 4 of the parent chain, this ethyl group has two methyl substituents. Thus, the name of the compound is 3−Ethyl−4, 7−dimethyl−4−(1,1−dimethylethyl) decane.
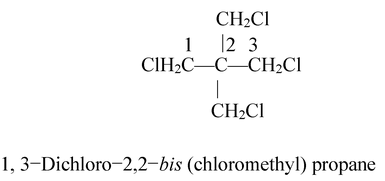
The numerical prefixes bis−, tris−, tetraki−, pentakisetc. are used to indicate a multiplicity of substituted substituent. The name of the substituted substituent is enclosed in parentheses. For example,
Nomenclature Of Compounds Having One Functional Group
It has been pointed out in the beginning that the functional groups (other than C = C or C ≡ C) present in the molecule are indicated by secondary suffixes. However, some of the functional groups are always regarded as substituents and are indicated by the prefixes. The various, rule which are followed while writing the name of the compounds having one functional group are described below.
Rule 1: Longest chain rule
Select the longest continuous chain of the carbon atoms as parent chain. The selected chain must include the carbon atoms involved in the functional groups like −COOH, −CHO, −CN, etc. or those which carry the functional groups like −OH, −NH 2 ,−Cl, −NO2 etc. The number of carbon atoms in parent chain decides the word root.
Rule 2 : Lowest number rule
The carbon atoms of the parent chain are numbered in such a way so that the carbon atom of the functional groups gets the lowest possible number. In case the functional group does not have the carbon atom, then the carbon atom of the parent chain attached to the functional group should get the lowest possible number.
Rule 3, Rule 4 and Rule 5 are used in the similar way as described in case of hydrocarbons earlier.
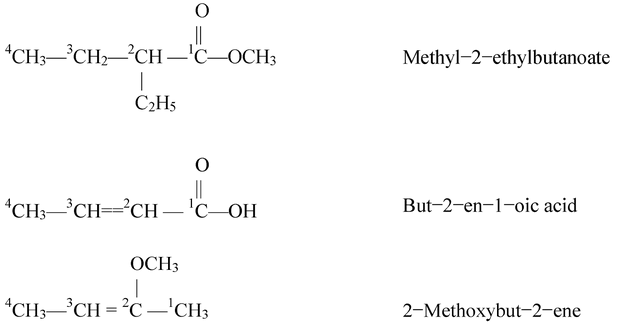
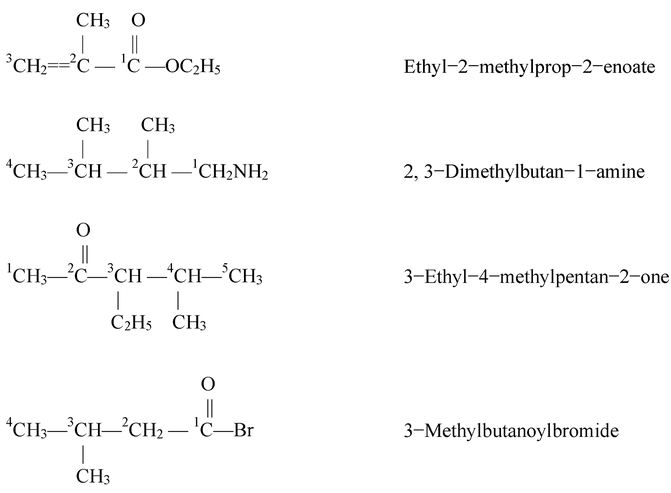
Some more examples of the compounds containing one functional group are being are below.
When there is a linear chain and two halide groups are present on the two corner, counting of carbon is done in increasing alphabetical order. For example,
Br − CH 2 CH 2 CH 2 CH 2 Cl
1−chloro−4−bromo butane is incorrect but correct name is 1−bromo−4−chloro butane
Naming The Compounds With More Than One Similar Functional Groups
If the organic molecule contains more than one similar functional groups, then in addition to various rules, the numerical prefixes di (for 2), tri(for 3) etc. are added before the secondary suffix which indicates the functional group. While adding such words, the vowel 'e' of the primary suffix is retained. For example,
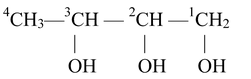
Here, word root is but, primary suffix is −ane and secondary suffix is ‘ol’. The −OH groups are positioned at carbon number 1,2 and 3. Thus, the name is,
Butane−1,2,3−triol or 1,2,3−Butanetriol
Some more examples of the compounds containing more than one similar functional group are given below.
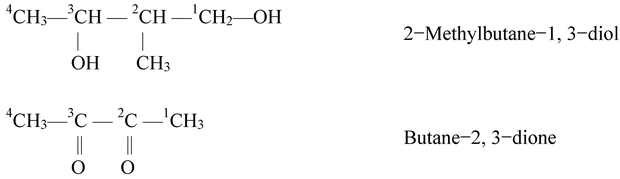
4 CH 2 = 3 CH — 2 CH = 1 CH 2 Buta−1,3−diene or 1,3−Butadiene









