Back Bonding
Chemical Bonding of Class 11
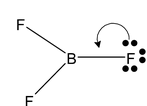
Sometimes surrounding atom to make up for electron deficiency in the central atom forms a π a -a bond which is one-sided i.e. shared electrons are contributed by the surrounding atom only which is having lone pair of electrons. Overlap involves a vacant orbital on the central atom and a filled orbital on the surrounding atom.
- Introduction
- Electrovalency
- Covalency
- General Properties Of Ionic And Covalent Bonds
- CO-Ordinate Covalency
- Hybridization
- Vsepr Theory (Valence Shell Electron Pair Repulsion Theory)
- Rule For Determination Of Total Number Of Hybrid Orbitals
- Resonance
- Rules For Writing Resonating Structures
- Deviation From Ideal Behavior
- Factors Governing Polarization And Polarisability (Fajan's Rule)
- Dipole Moment In Aromatic Ring System
- Percentage Of Iconic Character
- Hydrogen Bonding
- Types Of Hydrogen Bonding
- Effect Of Hydrogen Bonding
- Importance Of Hydrogen Bonding In Biological Systems
- Intermolecular-Forces
- Molecular Orbital Theory
- Inert Pair Effect
- Back Bonding
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5









