Covalency
Chemical Bonding of Class 11
COVALENCY
This type of valency involves sharing of electrons between the concerned atoms to attain the octet configuration with the sharing pair being contributed by both species equally. The atoms are then held by this common pair of electrons acting as a bond, known as covalent bond. If two atoms share more than one pair then multiple bonds are formed. Some examples of covalent bonds are

Sigma and Pi Bonding:
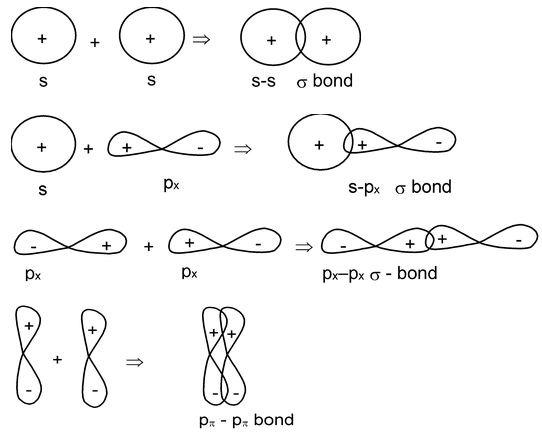
When two hydrogen atoms form a bond, their atomic orbitals overlap to produce a greater density of electron cloud along the line connecting the two nuclei. In the simplified representations of the formation of H2O and NH3 molecules, the O—H and N—H bonds are also formed in a similar manner, the bonding electron cloud having its maximum density on the lines connecting the two nuclei. Such bonds are called sigma bonds (σ-bond).
A covalent bond is established between two atoms having the maximum density of the electron cloud the line connecting the center of the bonded atoms is called a σ-bond. A σ-bond is thus said to possess a cylindrical symmetry along the internuclear axis.
Let us now consider the combination of two nitrogen atoms. Of the three singly occupied p-orbitals in each, only one p-orbital from each nitrogen (say, the px may undergo “head –on” overlap to form a σ-bond. The other two p-orbitals on each can no longer enter into a direct overlap. But each p-orbital may undergo lateral overlap with the corresponding p-orbital on the neighbour atom. Thus we have two additional overlaps, one by the two py orbitals, and the other by the two pz orbitals. These overlaps are different from the type of overlap in a σ-bond. For each set of p-orbitals, the overlap results in accumulation of charge cloud on two sides of the internuclear axis. The bonding electron cloud does no more posses an axial symmetry as with the σ-bond; instead, it possess a plane of symmetry. For the overlap of the pz atomic orbital, the xy plane provides this plane of symmetry; for the overlap of the py atomic orbitals, the zx plane serves the purpose. Bonds arising out of such orientation of the bonding electron cloud are designated as π-bonds. The bond formed by lateral overlap of two atomic orbitals having maximum overlapping on both sides of the line connecting the centres of the atoms is called a π-bond. A π-bond possess a plane of symmetry, often referred to as the nodal plane.