Fire extinguishers
Coal and Petroleum of Class 8
FIRE EXTINGUISHERS
A fire extinguisher is a device used for putting off fires. Though many kinds of fire extinguishers are available, carbon dioxide is the basic source for extinguishing fires in most of the extinguishers.
WORKING PRINCIPLE
Carbon dioxide gas is not a supporter of combustion. Being heavier than air, it forms a layer below air. Thus, CO 2 layer formed between the flames and air cuts off the contact between air and fire. As a result of this, the fire is prevented from spreading and is finally put off due to the shortage of the supply of oxygen to the fire.
TYPES:
The most important types of fire extinguishers in use are
- Soda acid fire extinguisher.
- Foam type fire extinguisher.
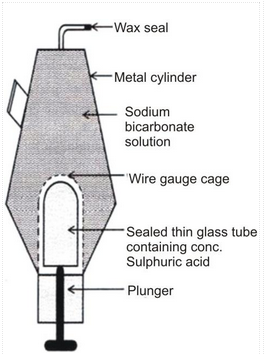
SODA ACID FIRE EXTINGUISHER:
A fire extinguisher consists of a metallic cylinder filled with a solution of sodium bicarbonate. At the bottom of the cylinder, a thin sealed glass tube containing concentrated sulphuric acid is placed. This tube is surrounded by a fixed wire guaze cage. A plunger with a sharp end is placed at the bottom of the cylinder in such a way that the sharp end is placed at the sealed thin glass tube, as shown in the above figure. On the top of the cylinder, a nozzle which is sealed with wax is provided.
The plunger is hit against the floor, The sharp tip of the plunger breaks the glass test tube. The acid in the test tube reacts with sodium bicarbonate solution to produce carbon dioxide gas. Carbon dioxide gas is forced out through the nozzle. The wax seal is broken, thus forcing CO 2 gas out through the nozzle. The fire is put off when the gas is directed against the fire.
LIMITATIONS:
This fire extinguisher is used to extinguish fire caused by solid inflammable materials only. In the case of extinguishing fires caused by inflammable liquids such as petrol it is rendered useless because the solution combing out of the cylinder, being denser than oil sinks in the oil and disables it to cut off the supply of air to the fire. It should not be used to extinguish the flames caused by electricity, as the solution is a good conductor of electricity.
FOAM TYPE EXTINGUISHER:
|
Foam type extinguisher is used to extinguish fire caused by inflammable liquids such as petrol, alcohol, diesel, etc. The construction and working of the extinguisher is similar to that of the extinguisher is similar to that of soda acid fire extinguisher. However, the solution of sodium bicarbonate contains saponin (a material which produces lot of foam). Sulphuric acid is replaced by aluminum sulphate solution. As a result the following reaction takes place, which helps in extinguishing fire.
Al 2 (SO 4 ) 3 + 6NaHCO 3 -------- 3Na 2 So 4 + Al(OH) 3 +6CO 2
LIMITATION: This fire extinguisher cannot be used to extinguish fire caused by electricity. |
|