
Petroleum
Coal and Petroleum of Class 8
PETROLEUM
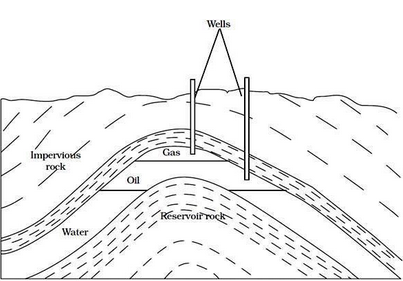
Petroleum is a natural resource from which diesel and petrol are obtained. Petrol is used in light automobiles while heavy motor vehicles like trucks, tractors run on diesel. Petroleum was formed from organism living in the sea.
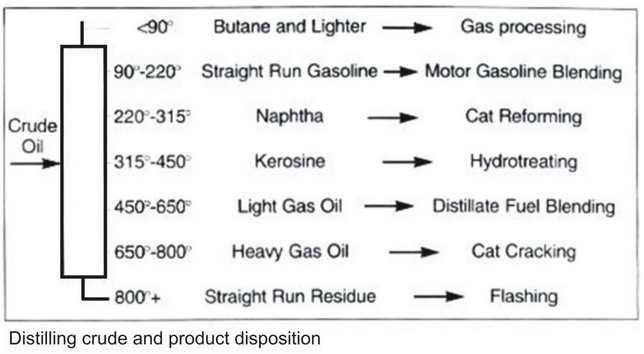
- Petroleum is a dark oily liquid. It has an unpleasant odour. It is a mixture of various constituents such as petroleum gas, petrol, diesel, lubricating oil, paraffin wax etc. The process of separating the various constituents of petroleum is known as refining. It is carried out in a petroleum refinery.
-
Many useful substances are obtained from petroleum and natural gas. These are termed as petrochemicals. These are used in the manufacture of detergents, fibers, polythene and other man made plastics. Hydrogen gas obtained from natural gas, is used in the production of fertilizers (Urea). Due to its great commercial importance, petroleum is also called " Black gold"
REFINING OF PETROLEUM
Petroleum is a complex mixture of organic liquids called crude oil and natural gas, which occurs naturally in the ground and was formed millions of years ago. Crude oil varies from oilfield to oilfield in colour and composition, from a pale yellow low viscosity liquid to heavy black 'treacle' consistencies.
As crude oil comes from the well it contains a mixture of hydrocarbon compounds and relatively small quantities of other materials such as oxygen, nitrogen, sulphur, salt and water. In the refinery, most of these non - hydrocarbon substances are removed and the oil is broken down into its various components, and blended into useful products

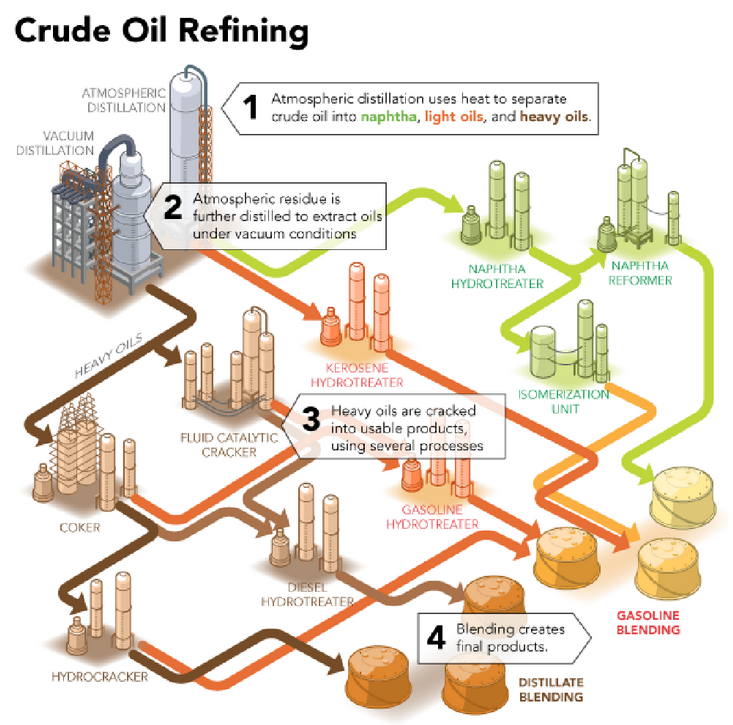
PRODUCTS OF PETROLUM REFINARY:
|
Product |
Description |
|
Refinery gas
|
|
|
Petrol |
|
|
Naphtha |
|
|
Kerosene |
|
|
Diesel |
|
|
Lubricating oil |
|
|
Fuel oil |
|
|
Residuals(Bitumen) |
|
NATURAL GAS:
Natural gas is a very important fossil fuel because it is easy to transport through pipes. Natural gas is stored under high pressure as compressed natural gas (CNG). CNG used for transport vehicles and power generation.
Natural gas is also used as a starting material for the manufacture of a number of chemicals and fertilizers. Natural gas has found in Tripura, Rajasthan, and Maharashtra and in Krishna Godavari delta.
COMBUSTION:
A chemical process in which a substance reacts with oxygen to give heat is called combustion. The substance that undergoes combustion is called to be a combustible. It is also called fuel. Example: petrol, kerosene. The fuel may be solid, liquid or gas. For combustion air is necessary. Combustion is a exothermic process i.e. substance releases heat on being combusted.
Combustion is defined as the burning of a fuel and oxidant to produce heat and/or work. It is the major energy release mechanism in the Earth and key to humankind's existence. Combustion includes thermal, hydrodynamic, and chemical processes. It starts with the mixing of fuel and oxidant, and sometimes in the presence of other species or catalysts. The fuel can be gaseous, liquid, or solid and the mixture may be ignited with a heat source. When ignited, chemical reactions of fuel and oxidant take place and the heat release from the reaction creates a self-sustained process. The combustion products include heat, light, chemical species, pollutants, mechanical work, and plasma. Sometimes, a low-grade fuel, e.g., coal, biomass, or coke, can be partially burned to produce high-grade fuel, e.g., methane. The partial burning process is called gasification. Various combustion systems, e.g., furnaces, combustors, boilers, reactors, and engines, are developed to utilize combustion heat, chemical species, and work.
e.g. C (Charcoal) + O
2
 CO
2
+ Heat + Light
CO
2
+ Heat + Light
CH
4
+ O
2
 CO
2
+ H
2
O + Energy
CO
2
+ H
2
O + Energy
The lowest temperature at which a substance catches fire is called ignition temperature. A combustible substance cannot catch fire or burn as long as its temperature is lower than its ignition temperature. The substances which have very low ignition temperature and can easily catch fire with a flame are inflammable substances. Example: petrol, alcohol, LPG (Liquefied petroleum gas) etc.
The head of the safety match is made from antimony trisulphide and potassium chlorate. The rubbing surface has powdered glass and a little red phosphorous. When the matches is struck against the rubbing surface, some red phosphorous get converted into white phosphorous which reacts with the potassium chlorate in the match ignite antimony trisulphide and start the combustion.
When there is a fire, break out, the fire bridge pours water on the fire. Water cools the combustion material so that its temperature is brought below its ignition temperature thus, preventing the fire from spreading.
The job of the fire extinguisher is to cut off the supply of the air, or to bring down the temperature of the fuel or both. The most common fire extinguisher is water but it is not suitable for involving oil and petrol. For fires involving electrical equipment and inflammable materials, carbon dioxide (CO 2 ) is the best extinguisher, which is stored at the high pressure as a liquid in cylinders. Also, near the fire CO 2 can be given off by using chemical like sodium bicarbonate, potassium bicarbonate etc.To score More in your class 8 refer NCERT solutions for class 8 .










