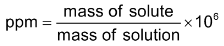Concentration Units
Liquid Solution of Class 12
Concentration Units
The concentration of a solute is the amount of solute dissolved in a given quantity of solvent or solution. The quantity of solvent or solution can be expressed in terms of volume or in terms of mass or molar mass. Thus there are several ways of expressing the concentration of a solution.
(a) Molarity (M): Moles of solute present in one litre solution.

(b) Molality (m): Moles of solute present in one kilogram of solvent.
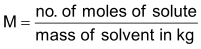
(c) Normality (N): No. of equivalents present in one litre solution.
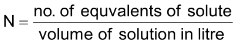
(d) Mole fraction: The mole fraction of a component substance A(XA) in a solution is defined as the moles of component substance divided by the total moles of solution.
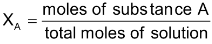
Mass percent: The mass percent of a component A in solution is defined as
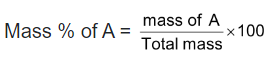
Part per million (PPM): It is defined as the parts of given component in one million parts of solution. Mathematically