
Solubility Of Gases
Liquid Solution of Class 12
Solubility Of Gases
We are familiar that gases are completely miscible with each other. Gases also dissolve in liquids and solids. For example, soda-water contains carbon dioxide dissolved in water under high pressure. Oxygen is sufficiently soluble in water to allow survival of aquatic life in lakes, rivers and oceans. An example of dissolution of gas in a solid is the solubility of hydrogen gas in palladium.
The solubility of a gas in a liquid is determined by several factors. In addition to the nature of the gas and the liquid, solubility of the gas depends on the temperature and pressure of the system. The solubility of a gas in a liquid is governed by Henry’s law which states that the solubility of a gas in a liquid is directly proportional to the pressure of the gas. Dalton, a contemporary of Henry, also concluded independently that the solubility of a gas in a liquid solution is a functional of the partial pressure of the gas. If we use the mole fraction of the gas in the solution as a measure of its solubility, then:
Mole fraction of the gas in a solution is proportional to the partial pressure of the gas.
Or, partial pressure of the gas in solution = KH × mole fraction of the gas in solution
Here KH is Henry’s law constant
Or p = KHx
If we draw a graph between partial pressure of the gas versus mole fraction of the gas in solution, then we should get a plot of the type as shown in figure.
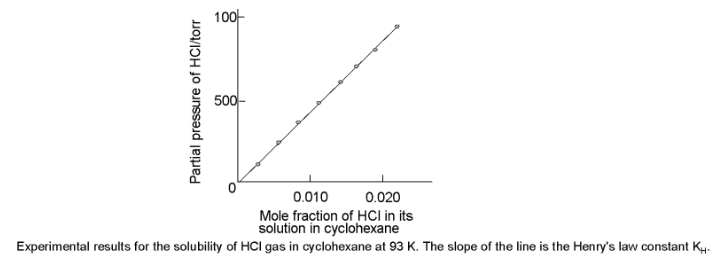
Different gases have different KH values at the same temperature. This suggests that KH is a function of the nature of the gas. Table gives KH values of some common gases at specified temperature
Values of Henry’s law constant (KH) for some selected gases in water
|
Gas |
Temp/K |
KH/kbar |
|
He |
293 |
144.97 |
|
H2 |
293 |
69.16 |
|
N2 |
293 |
76.48 |
|
N2 |
303 |
88.84 |
|
O2 |
293 |
34.86 |
|
O2 |
393 |
46.82 |
It is obvious from figure that the higher the value of KH at a given pressure, the lower is the solubility of the gas in the liquid. It can be seen from table that KH value for both N2 and O2 increases with increase in temperature indicating that solubility of gases decreases with increase of temperature. It is due to this reason that aquatic species are more comfortable in cold waters rather than warm waters.
Henry’s law finds several applications in industry and explains some biological phenomenon. Notable among these are:
(i) To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
(ii) To minimize the painful effects accompaynig the decompression of deep sea divers, oxygen diluted with less soluble helium gas is used as breathing gas.
(iii) In lungs, where oxygen is present in air with high partial pressure, haemoglobin combines with oxygen to form oxyhaemoglobin. In tissues where partial pressure of oxygen is low, oxyhaemoglobin releases oxygen for utilization in cellular activities.
