Introduction
Liquid Solution of Class 12
|
Illustration 37. |
|
||||
|
(A) |
|
(B) |
|
||
|
(C) |
|
(D) |
None |
||
|
Solution: |
|
||||
|
Hence (C) is correct. |
|||||
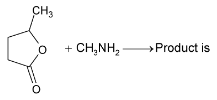
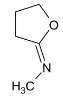
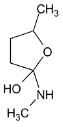
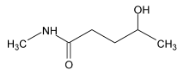
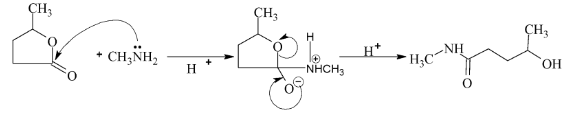
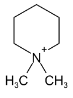
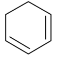
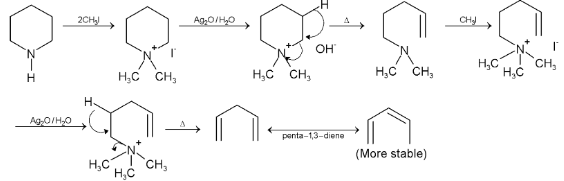
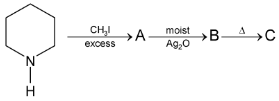
Illustration 38. Identify the end product (C)
|
(A) |
|
(B) |
|
|
(C) |
|
(D) |
|
|
Solution: |
|
|
Hence (A) is correct. |
Amino acids and Proteins:
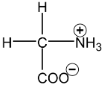
Amino acids are derivatives of carboxylic acids in which a hydrogen atom at the  is replaced by an amino group. Amino acids are obtained by hydrolysis of proteins. The amino acids can be represented by
is replaced by an amino group. Amino acids are obtained by hydrolysis of proteins. The amino acids can be represented by
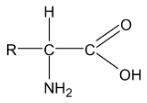
About twenty five amino acids are obtained from proteins, of which about ten are essential, i.e. a deficiency in any one prevents growth in young animals and may even cause death. Simplest amino acids are
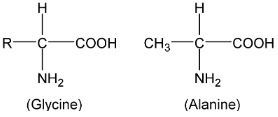
|
Alanine and all amino acids except glycine contain an asymmetric carbon atom and exhibit optical isomerism (naturally occurring amino acids are laevorotatory). Amino acids contain amino and carboxyl groups and show the properties of both groups. Amino acids have high melting points and are usually water soluble. They usually exist as inner salts or zwitter ions or dipolar ion (a species containing both positive and negative charges). |
|
Amino acids are amphoteric.
Amino acids are linked together by peptide linkages.
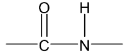
Peptides are condensation products of two or more amino acids through their amino and carboxylic groups involving elimination of water molecules. Amino acid polymers with relative molecular masses 10,000 or less are called polypeptides. Those with larger molecular masses are called proteins e.g. egg albumen 40,000.
Illustration 39. Explain why does glycine exist as a Zwitter ion but anthranilic acid does not.
Solution: The ⎯COOH group of glycine releases H+ ion which is accepted by ⎯NH2 group. Thus glycine exists as a zwitter ion.
However, the electron withdrawing nature of ⎯COOH group reduces electron density on N in anthranilic acid. Thus nitrogen atom of amino group fails to accepts the proton released by the ⎯COOH group. That is why anthranilic acid does not exist as zwitter ion.
Test for Proteins:
Presence of proteins can be shown by warming the suspected protein with dilute NaOH solution and CuSO4 solution. A violet colouration confirms presence of proteins.
Methods of preparation of amino acids:
(i) Amination of 
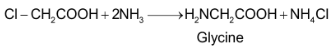
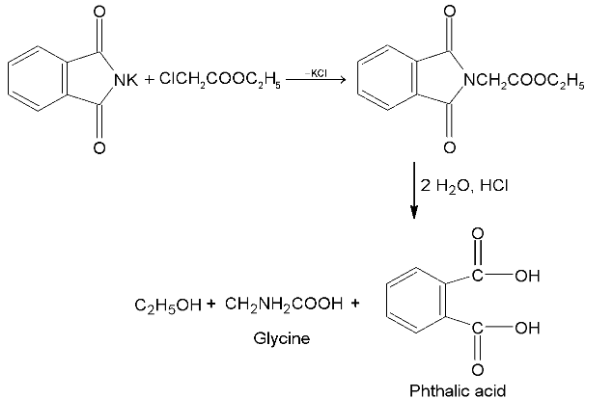
(ii) Gabriel phthalimide synthesis:
 acid or ester combines with potassium phthalimide. Product on hydrolysis gives
acid or ester combines with potassium phthalimide. Product on hydrolysis gives  amino acid
amino acid
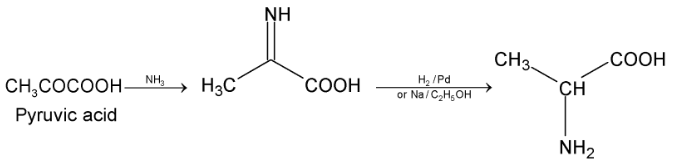
(iii) Knoop synthesis:
 keto acid on treatment with NH3 forms an imine which on catalytic reduction gives amino acid.
keto acid on treatment with NH3 forms an imine which on catalytic reduction gives amino acid.
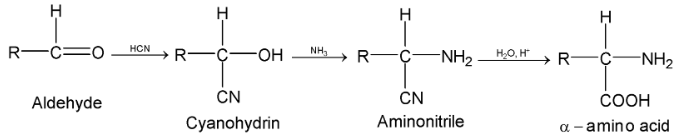
(iv) Strecker synthesis:
Aldehydes react with HCN and ammonia or NH4CN and the product on hydrolysis yields  acids.
acids.
Some of the reactions given by amino acids are as follows:
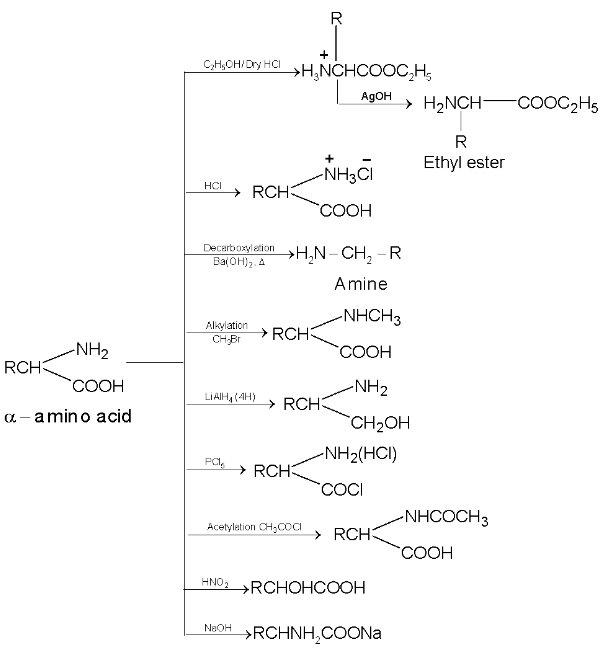
Action of heat:
 amino acids lose two molecules of water and form cyclic amides.
amino acids lose two molecules of water and form cyclic amides.
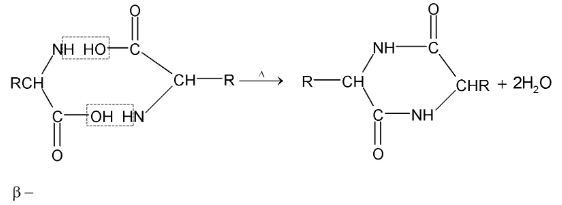
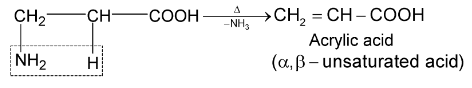
ß amino acids lose a molecule of ammonia per molecule of amino acid to yield 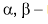 unsaturated acids.
unsaturated acids.