Interconversion Of States Of Matter
Matter is our surrounding of Class 9
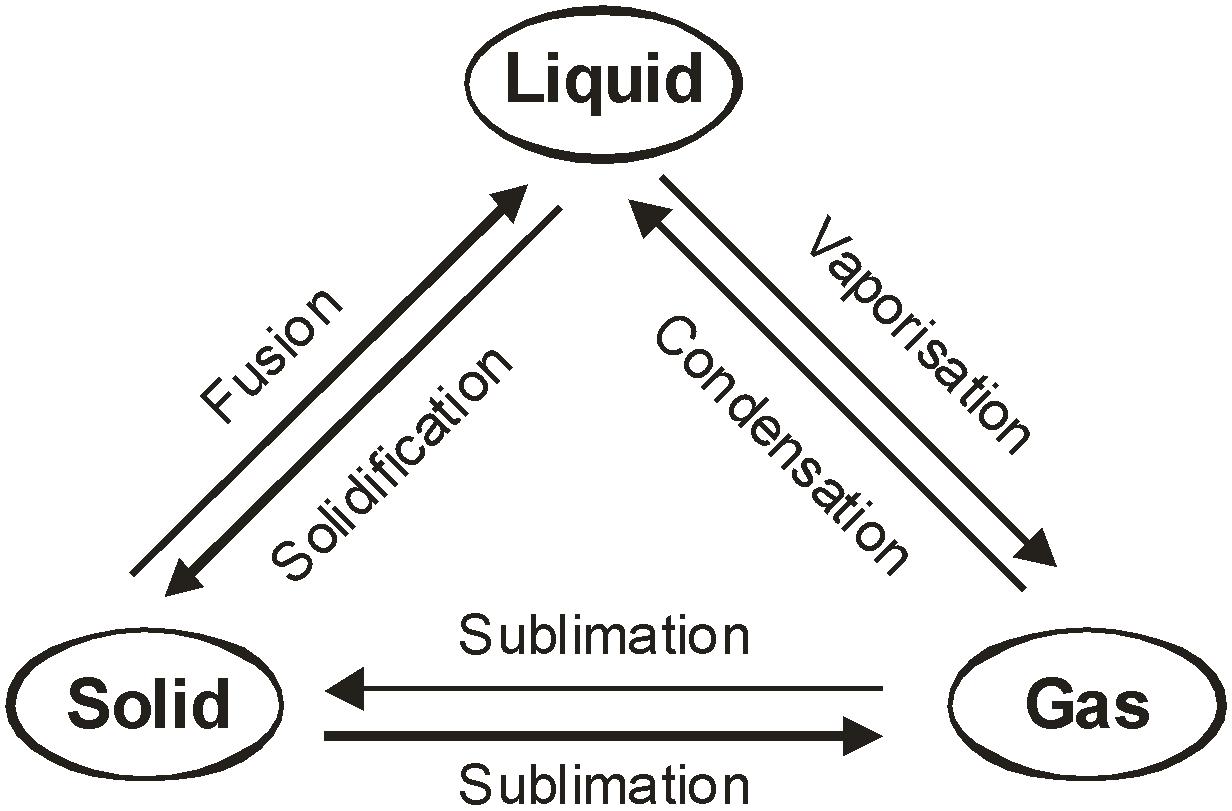
INTERCONVERSION OF STATES OF MATTER
The phenomenon of change of matter form one state to another state and back to original state, by altering the conditions of temperature and pressure, is called interconversion of matter.
The various states of matter can be interchanged into one another by altering the conditions of:
(a)Temperature (b) Pressure.
Altering the Temperature of Matter :
(i) Interconversion of solid into liquid and melting : The solids can be converted into liquids by heating them. Similarly liquids can be cooled to form solids.
e.g. : Ice at 0 o C changes into water at 0 o C, when heat energy is supplied to it. The water at 0 o C changes into ice at 0 o C on freezing.
- Melting or Fusion : The process due to which a solid changes into liquid state by absorbing heat energy is called melting or fusion.
- Freezing or Solidification : The process due to which a liquid changes into solid state by giving out heat energy is called freezing or solidification.
- Melting Point : The constant temperature at which a solid changes into liquid state by absorbing heat energy is called it melting point.
- Freezing Point : The constant temperature at which a liquid changes into solid state by giving out heat energy is called freezing point.
The numerical value of freezing point and melting point is same. Melting point of ice = Freezing point of water = 0 o C (273.16 K).
Explanation: On increasing the temperature of solids, the kinetic energy (K.E.) of particles increases. Due to increases in K.E., the particles start vibrating with greater speed. The energy supplied overcomes the force of attraction between the particles. Then, the particles leave their fixed positions and start moving freely and thus solid melts.
Latent heat: The amount of heat required to change the state of matter from one state to another without rise in temperature is known as latent heat of that substance.
Latent heat is of two types:
- Latent heat of fusion: The amount of heat required to change the state of matter from solid state to liquid state without rise in temperature at its melting point is known as latent heat of fusion.
- Latent heat of vaporisation: The amount of heat required to change the state of matter from liquid state to gaseous state without rise in temperature at its boiling point is known as latent heat of vaporisation.
- Interconversion of liquid into gaseous state and vice versa : Liquids can be converted into gases by heating them. Similarly, gases can be converted into liquids by cooling them.
e.g. Water at normal pressure changes into gas (steam) at 100 o C by absorbing heat. Steam at 100 o C changes into water by giving out energy.
- Boiling or Vaporisation: The process due to which a liquid changes into gaseous state by absorbing heat energy is called boiling.
- Condensation or Liquefaction: The process due to which a gas changes into liquid state by giving out heat energy is called condensation.
- Boiling point: The constant temperature at which a liquid rapidly changes into gaseous state by absorbing heat energy at atmospheric pressure is called boiling point.
- Condensation Point: The constant temperature at which a gas changes into liquid state by giving out heat energy at atmospheric pressure is called condensation point.
The numerical value of condensation point and boiling point is same. Condensation point of vapour (water) = Boiling point of water = 100 o C (373 K).
Explanation: When heat is supplied to water, particles start moving faster. At a certain temperature, a point is reached when the particles have enough energy to break the forces of attraction between the particles. At this temperature the liquid starts changing into gas.
Latent heat of vaporisation: The amount of heat which is required to convert 1 kg of the liquid (at its boiling point) to vapour or gas without any change in temperature. Latent heat of vaporisation of water = 22.5 × 10 5 J/kg.
Particles in steam, that is water vapour at 373 K have more energy than water at the same temperature. Because steam have absorbed extra energy in the form of latent heat of vaporisation.
Direct interconversion of solid into gaseous state and vice versa:
SUBLIMATION
Sublimation is the process of conversion of a solid directly into a gas or vice-versa without changing into liquid state.
Experiment to demonstrate sublimation: Take some ammonium chloride (NH 4 Cl) in a china dish, and cover it with an inverted funnel as shown in figure. Plug the stem of funnel with cotton. Now heat slowly.
Observation and Discussion: Ammonium chloride, will convert into vapours which will deposit on the inner side of the funnel as sublimate. The vapours in turn, condense on the cooler portions of the funnel to give pure NH 4 Cl.
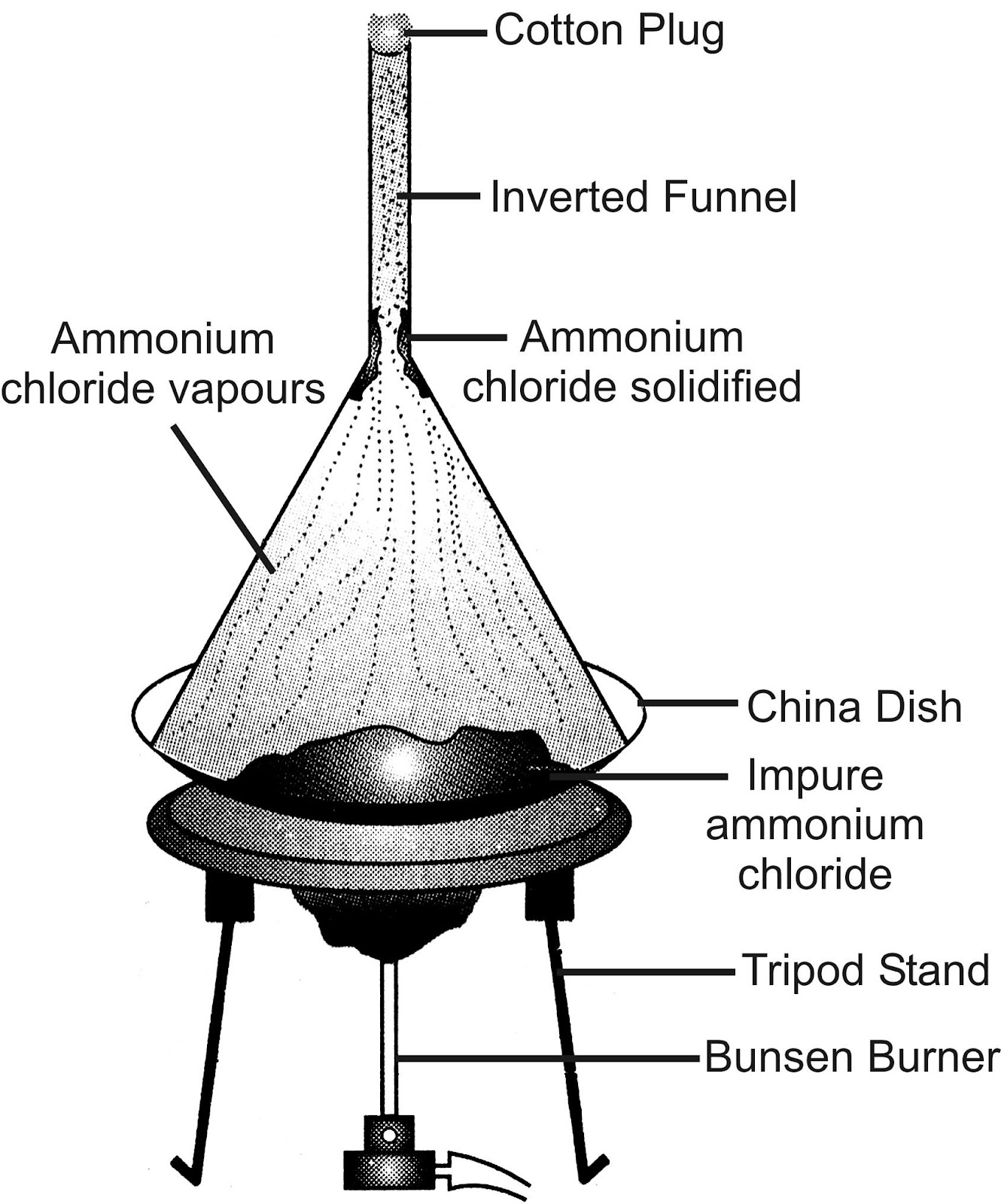
Sublimation of ammonium chloride
Conclusion: A change of state directly from solid to gas without changing into liquid (or vice-versa) is called sublimation.
EFFECT OF CHANGE OF PRESSURE
The effect of pressure on the states of matter can be discussed by the following experiment:
Experiment: Take a gas in a cylinder and apply pressure by pushing the piston down as shown in figure.
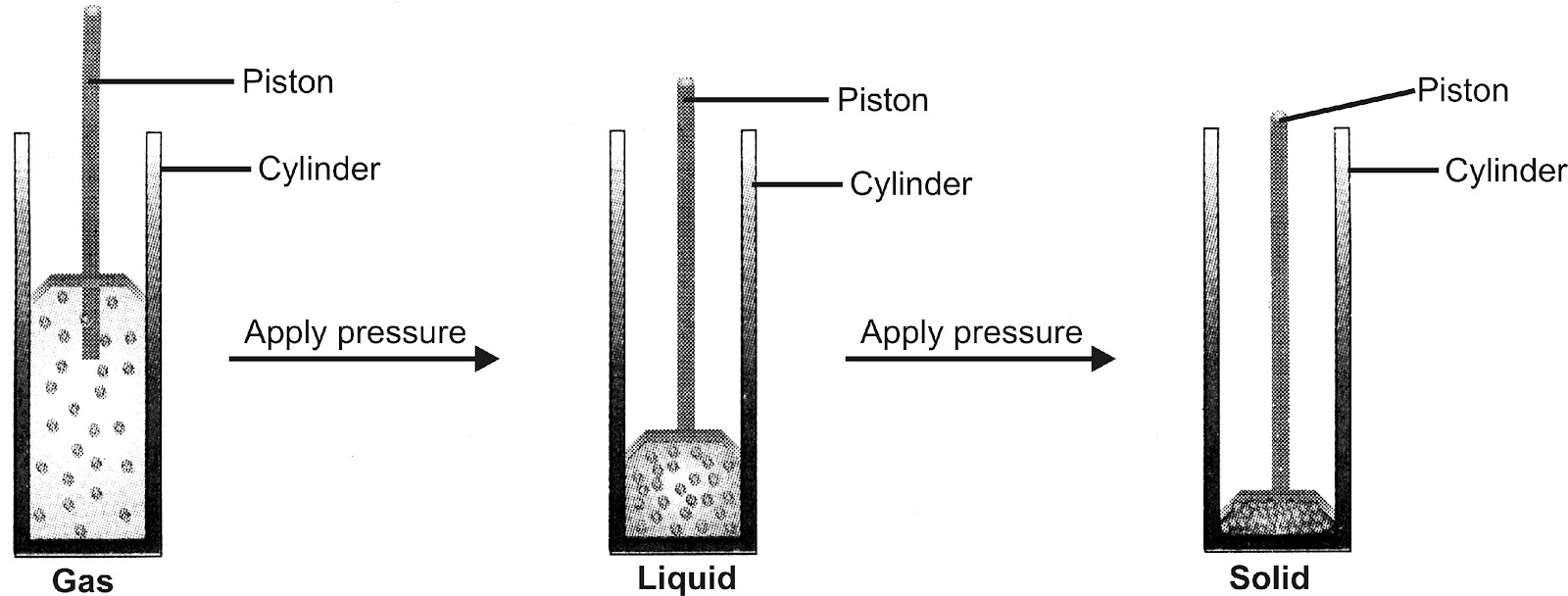
(a) (b) (c)
By applying pressure, particles of a gas come close together
Observation: A gas can be first liquified and then converted into solid.
Liquification of gas : A gas can be liquified by applying pressure or by lowering the temperature. For every gas, there is a minimum temperature above which gas cannot be liquified by applying pressure. This temperature is called “critical temperature”. The minimum pressure which is required to liquified a gas at critical temperature is called “critical pressure”.
Discussion: When the particles of fluid are present under low pressure, they are in the gaseous state as shown in the figure (a). When some high pressure is applied on the gas, the forces of attraction between gas particles become so high that they bind the gas particles together to form the liquid state [figure (b)]. Ultimately under very high pressure, the forces of attraction become so strong that the liquid may change into the solid state [figure (c)].
For example, CO 2 gas can be liquified easily either by applying pressure or by reducing the temperature. However, CO 2 is cooled (by reducing temperature) under high pressure, it can be directly converted into solid CO 2 called ‘dry ice’.
Conclusion: From above discussion, It is clear that a gas can be liquified by increasing pressure and decreasing temperature hence, it follows that both pressure and temperature determine the state of a substance, whether, it will be a solid, liquid or gas. The entire change has been represented as below: