Structural Polysaccharides
Molecules of Cell of Class 11
Structural Polysaccharides
Fibrous polysaccharides that are employed in the formation of cell walls of plants, fungi and exoskeleton of arthropods, e.g. cellulose, fungus cellulose and chitin.
Cellulose
About 50% of the carbon found in plants is in cellulose and it is the most abundant organic molecule on Earth.
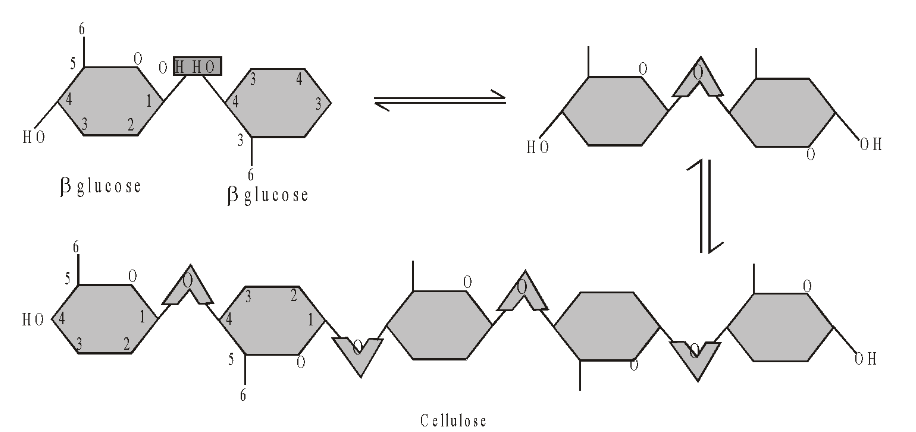
A polymer of glucose. When two molecules of glucose line up, the –OH group on carbon atom 1 can only line up alongside the –OH group on carbon atom 4 if one of the molecules is rotated at 180° to the other. This is because the –OH group on carbon atom 1 projects below the ring and the –OH group on carbon atom 4 projects above the ring. This rotation of successive residues is the underlying reason why cellulose has a different structure to starch.
Structural component of all plant cell walls, making up about 20-40% of the wall on average. Consists of long chains of glucose residues with about 10000 residues per chain. The 1,4 linkages make the chains straight in contrast to starch where 1, 4 linkage cause the chains to be curved.
Hydroxyl groups (–OH) project outwards from each chain in all directions and form hydrogen bonds with neighbouring chains. This cross-linking binds the chains rigidly together. The chains associate in groups of about 60 to 70 to form microfibrils, which are arranged in larger bundles to form macrofibrils which have tremendous tensile strength. In cell walls the macrofibrils are arranged in several layers, in a glue-like matrix made of other polysaccharides.
Plant cells are therefore wrapped in several layers of cellulose. This prevents the cells from bursting when water enters by osmosis and also helps to determine the shapes of cells, since the direction in which they expand depends on the way the layers are arranged. Despite their combined strength, the layers are fully permeable to water and solutes, an important property in the functioning of plant cells.
Cellulose is also an important foodsource for some animals, bacteria and fungi.
The enzyme cellulase, which catalyses the digestion of cellulose to glucose, is relatively rare in nature and most animals, including humans, cannot utilise cellulose despite its being an abundant and potentially valuable source of glucose. Ruminant mammals like the cow, however have bacteria living symbiotically in their guts which digest cellullose.
Abundance of cellulose and its relatively slow rate of breakdown in nature have ecological implications because it means that substantial quantities of carbon are ‘locked up’ in this substance, and carbon is one of the chief materials required by living organisms.
Commercially, cellulose is extremely important. e.g. used to make cotton goods and is a constituent of paper and Sellotape.
Rayon and Cellophane are mainly formed of cellulose xanthate.
Cellulose Acetate fibres yield wrinkle-proof, moth proof tericot and double knits. Cigarette filters are also formed from these fibres. Other uses of cellulose acetate is in plastics, shatter proof glass and transparent photographic films.
Cellulose Nitrate (Nitrocellulose) yields propellant explosives. Gun cotton is fully nitrated cellulose. Smokeless ammunition powder is a mixture of higher and lower nitrates of cellulose. Carboxymethyl Cellulose : It is semisynthetic gum used in detergents for removing dust (hence extra brightening effect), sizing of paper and textiles, stabilisation of latex paints, smoothening reagent in cosmetics, medicines, ice creams and other foods.
Chitin
A structural polysaccharide that occurs in some fungi, where its fibrous nature contributes to cell wall structure, and in some animal groups, particularly the arthropods, it forms an essential part of the exoskeleton.
Structurally it is identical to cellulose except that the hydroxyl (–OH) group at carbon atom 2 is replaced by –NH.CO.CH 3 . Linear unbranched polymer of N-acetyl glucosamine.
Forms bundles of long parallel chains like cellulose cross linked by hydrogen bonds.
The second most abundant organic material.
It is both soft and leathery. Where hardness is required, chitin is impregnated with calcium carbonate and fibrous proteins.
Impermeable to water and prevents loss of water through evaporation.
Murein
Acts as the strengthening material of bacterial cell walls. It is similar in structure to chitin, containing nitrogen like chitin.
Mucopolysaccharides
Heteropolysaccharides of high molecular weight that are gelatinous in consistency, functioning in lubrication and components of sticky substances in organisms. Vary from pectins of cell wall, calcium pectate of middle lamella, gums mucilages, slimes and phycocolloids to hyaluronic acid, chondroitin sulphate, keratin sulphate, heparin, etc. They often contain amino sugars and sugar acids.
Various types of function as follows :
Mucilage Sheaths occur over aquatic plants, cyanobacteria (blue-green algae) and bacteria and protect the organisms from desiccation, rotting effect of water, epiphytic growth and toxic chemicals. Mucilage also occurs in the unripe fruits of Okra (Lady finger, vern Bhindi) especially around the seeds.
Pectin occurs in cell wall. Calcium pectate is found in middle lamella. Pectin is made of galactose, galacturonic acid and arabinose. Pectin compounds of middle lamella help in cementing two adjacent cells,pectins of cell wall determine its hydration and orientation of cellulose microfibrils during growth. Pectin compounds are commercial jellying agents.
Psyllium (Isbgol) powdered colloidal mucilage obtained from the seed husks of Plantago ovata (Plantain, vern, Isbgol), used as a purgative.
Aloe Gel is gelatinous pulp obtained from fleshy leaves of Aloe barbadensis. Gives relief when applied over inflamed areas and also added in shampoos, conditioners, hand lotions and sun screen creams.
Agar-Agar is cell wall mucopolysaccharide of some red algae (e.g. Gelidium, Gelidiella, Gracilaria). The phycocolloid is formed of D-galactose 3-6 anhydro L-galactose with sulphate at every tenth position.
Agar is used as culture medium, thickener, stabilizer and as laxative. Related compound is carrageenin (from Chondrus) as emulsifier and clearing agent.
Hyaluronic Acid is a thread-like heteropolysaccharide formed by alternating unit of D-glucuronic acid and N-acetylglucosamine. Hyaluronic acid acts as cement between adjacent animal cells. It occurs in the matrix of connective tissue, around ovum and in various lubricating fluids like cerebrospinal fluid, synovial fluid of bone joints and viscous solution in vitreous humour.
Keratosulphate (Keratin sulphate) is formed from N-acetylglucosamine, galactose and sulphate. It provides both strength and flexibility to skin, connective tissues, cartilage and cornea. It is also complexed with hyaluronic acid in forming proteoglycan.
Chondroitin Sulphate is a linear polymer of sulphated N-acetylgalactosamine alternating with glucuronic or iduronic acid. It is able to bind water and provide support to connective tissue against compression.
The complex also occurs in skin, tendon and cartilage.
Heparin is a highly sulphated mucopolysaccharide made of alternate units of glucosamine and glucuronic acid. Heparin is mainly produced by liver and mast cells. It is most abundant in blood and connective tissue. Inhibits blood coagulation inside the blood vessels and used as anticoagulant.
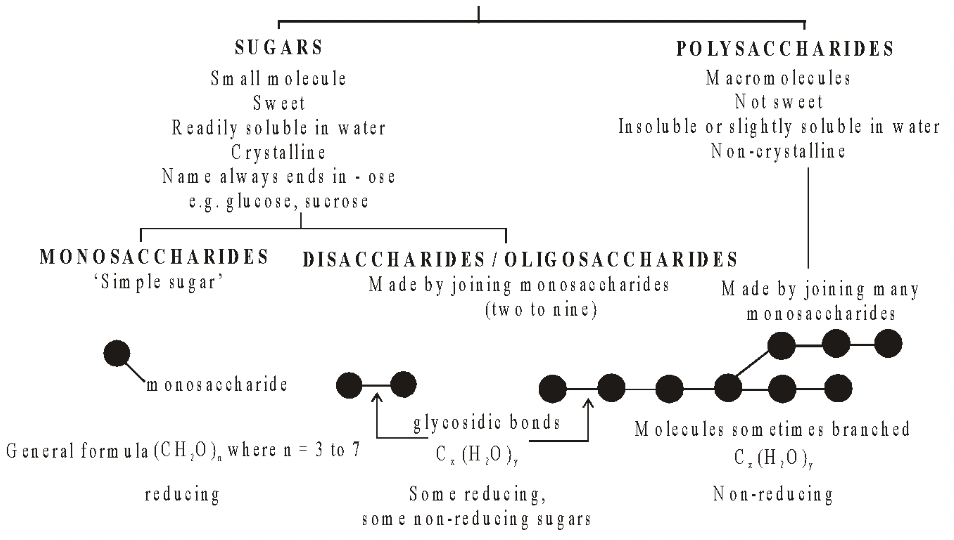
Carbohydrates are substances which contain the elements carbon, hydrogen and oxygen and have the general formula Cx(H 2 O)y, where x and y are variable numbers; their name (hydrate of carbon) is derived from the fact that hydrogen and oxygen are present in the same proportions as in ‘water’ namely two hydrogen atoms to one oxygen atom.
They are aldehydes or ketones and all contain several hydroxyl groups. Their chemistry is determined by these groups. e.g. aldehydes are very easily oxidised and hence are powerful reducing agents.
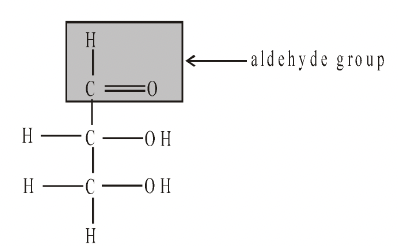
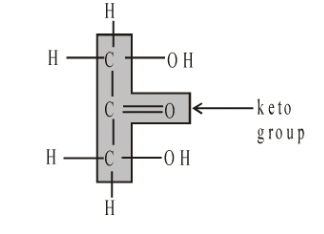
Glyceraldehyde Dihydroxyacetone
In monosaccharides, all the carbon atoms except one have a hydroxyl group attached. The remaining carbon atom is either part of an aldehyde group, in which case the monosaccharide is called an aldose or aldo sugar, or is part of a keto group, when it is called a ketose or keto sugar. Thus all monosaccharides are aldoses or ketoses. The two simplest monosaccharides are the trioses-glyceraldehyde and dihydroxyacetone. Glyceraldehyde has an aldehyde group and dihydroxyacetone has a keto group. In general, aldoses, such as ribose and glucose, are more common than ketoses, such as ribulose and fructose.
Carbohydrates are divided into three main classes, Monosaccharides, Oligosaccharides and Polysaccharides.