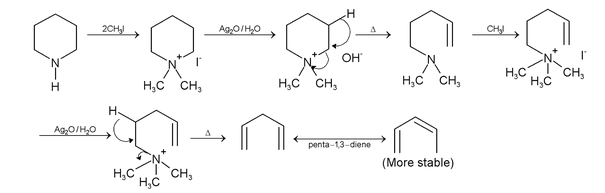
Cyanides And Isocyanides
Nitrogen and Other P block Elements of Class 12
Cyanides And Isocyanides
Both alkyl cyanides (RCN) and alkyl isocyanides (RNC) are organic derivatives of hydrocyanic acid HCN. Alkali cyanides are ionic
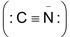 and cyanide ion is ambident in nature (can form covalent bond either from carbon or nitrogen).
and cyanide ion is ambident in nature (can form covalent bond either from carbon or nitrogen).
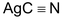 is covalent, hence lone pair on nitrogen is mainly available for covalent bond formation, resulting in predominant formation of isocyanides.
is covalent, hence lone pair on nitrogen is mainly available for covalent bond formation, resulting in predominant formation of isocyanides.
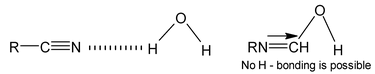
Illustration 34. How would you account for the fact that alkyl cyanides are soluble in water but alkyl isocyanides are insoluble in water?
Solution: Alkyl cyanides possess the tendency to form H – bonding with water which is absent with isocyanides
Methods of preparation of Cynaides :
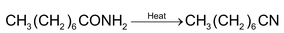
1. Dehydration of Amides:
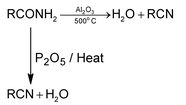
High molecular weight acid amides are dehydrated to the corresponding cyanide by heat alone.
2. From RX:

This method is satisfactory only if R is 1º or 2º group. If it is 3º group, then it is converted into alkene.

3. By Grignard’s reagent and Cyanogen chloride reaction:

This is best method for preparing 3º alkyl cyanides.

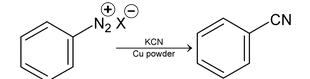
4. From Diazonium salt
Properties:
1. Lower members are liquids which are soluble in water but solubility decreases as molecular weight increases.
2. Since alkyl cyanides cannot form intermolecular hydrogen bonds, hence they are much more volatile than their corresponding acids.
Reactions:
1. Hydrolysis:
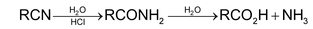
Partial hydrolysis to acid amides is achieved with alkaline H2O2.
Alkaline hydrolysis of
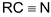 gives sodium salt.
gives sodium salt.
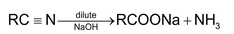
2. Reduction:
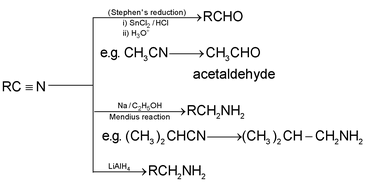
3. With Grignard’s reagent (ketone formation):
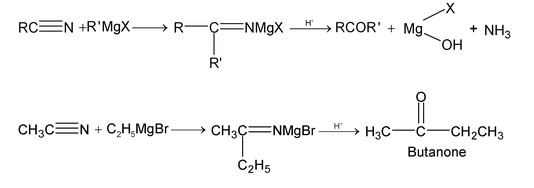
4. With alcohol:
When a solution of RCN in an alcohol is heated with conc. H 2 SO 4 or HCl, ester is obtained.
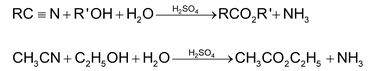
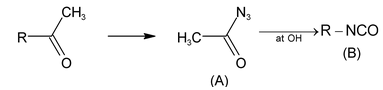
Illustration 35. The products (A) and (B) formed in the given reaction is:
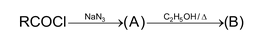
(A) RCON and RCN (B) RCN and RNC
(C) RCON 3 and RCN (D) RCON 3 and RNCO
|
Solution: |
|
|
Hence (C) is correct. |
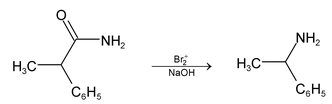
Illustration 36. Alkanamide which on Hoffmann’s reaction gives 1−phenyl ethyl amine is:
(A) 2−phenylpropanamide (B) 3−phenylpropanamide
(C) 2−phenylethanamide (D) N−phenylethanamide
|
Solution: |
|
|
Hence (A) is correct. |
Methods of Preparation of Isocyanides:
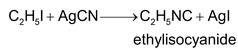
1. By heating an alkyl iodide with AgCN in aqueous ethanolic solution

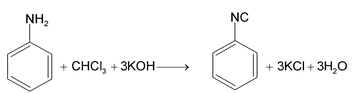
2. By carbylamine reaction
Heating a mixture of a1º mine and chloroform with ethanolic potassium hydroxide
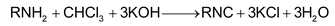
Mechanism proceeds via intermediate formation of dichloromethylene or, dichloro carbene produced from chloroform in alkaline solution. (Via α-elimination)
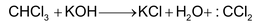
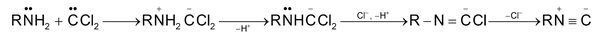
Properties:
1. Alkyl isocyanides are poisonous, unpleasant smelling, with lower boiling points than isomeric cyanides.
2. RNC are not very soluble in water, nitrogen atom not having a lone pair of electrons available for hydrogen bonding.
Reactions:
1. Hydrolysis:
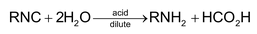
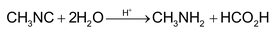
RNC are not hydrolysed by alkalis.
2. Reduction:
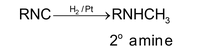

3. When alkyl isocyanides are heated for a long time, they arrange to form cyanide


4. With non metals:
(i)

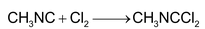
(II)


5. Oxidation with HgO:
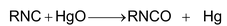

|
Illustration 37. |
|
||||
|
(A) |
|
(B) |
|
||
|
(C) |
|
(D) |
None |
||
|
Solution: |
|
||||
|
Hence (C) is correct. |
|||||
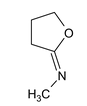
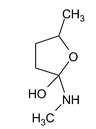
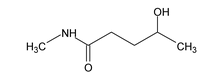
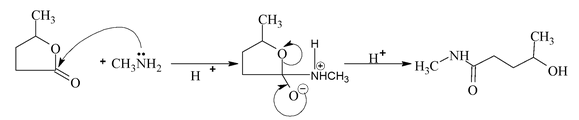
Illustration 38. Identify the end product (C)
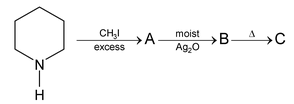
|
(A) |
|
(B) |
|
|
(C) |
|
(D) |
|
|
Solution: |
|
|
Hence (A) is correct. |
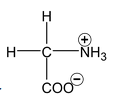
Amino acids and Proteins:
Amino acids are derivatives of carboxylic acids in which a hydrogen atom at the
 is replaced by an amino group. Amino acids are obtained by hydrolysis of proteins. The amino acids can be represented by
is replaced by an amino group. Amino acids are obtained by hydrolysis of proteins. The amino acids can be represented by
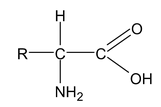
About twenty five amino acids are obtained from proteins, of which about ten are essential, i.e. a deficiency in any one prevents growth in young animals and may even cause death. Simplest amino acids are
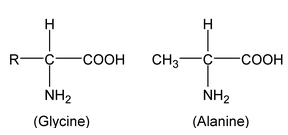
|
Alanine and all amino acids except glycine contain an asymmetric carbon atom and exhibit optical isomerism (naturally occurring amino acids are laevorotatory). Amino acids contain amino and carboxyl groups and show the properties of both groups. Amino acids have high melting points and are usually water soluble. They usually exist as inner salts or zwitter ions or dipolar ion (a species containing both positive and negative charges). |
|
Amino acids are amphoteric.
Amino acids are linked together by peptide linkages.
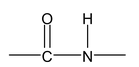
Peptides are condensation products of two or more amino acids through their amino and carboxylic groups involving elimination of water molecules. Amino acid polymers with relative molecular masses 10,000 or less are called polypeptides. Those with larger molecular masses are called proteins e.g. egg albumen 40,000.
Illustration 39. Explain why does glycine exist as a Zwitter ion but anthranilic acid does not.
Solution: The ⎯COOH group of glycine releases H+ ion which is accepted by ⎯NH 2 group. Thus glycine exists as a zwitter ion.
However, the electron withdrawing nature of ⎯COOH group reduces electron density on N in anthranilic acid. Thus nitrogen atom of amino group fails to accepts the proton released by the ⎯COOH group. That is why anthranilic acid does not exist as zwitter ion.
Test for Proteins:
Presence of proteins can be shown by warming the suspected protein with dilute NaOH solution and CuSO4 solution. A violet colouration confirms presence of proteins.
Methods of preparation of amino acids:
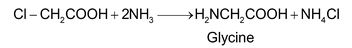
(i) Amination of

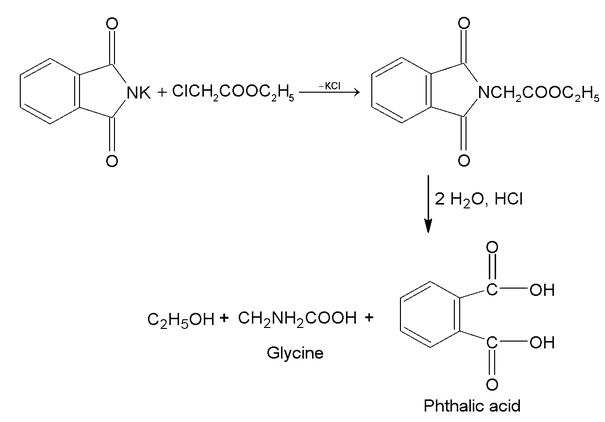
(ii) Gabriel phthalimide synthesis:
 acid or ester combines with potassium phthalimide. Product on hydrolysis gives
acid or ester combines with potassium phthalimide. Product on hydrolysis gives
 amino acide
amino acide
(iii) Knoop synthesis:
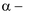 keto acid on treatment with NH3 forms an imine which on catalytic reduction gives amino acid.
keto acid on treatment with NH3 forms an imine which on catalytic reduction gives amino acid.
(iv) Strecker synthesis:
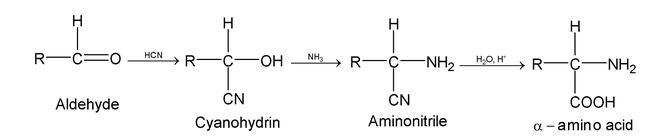
Aldehydes react with HCN and ammonia or NH
4
CN and the product on hydrolysis yields
 amino acids.
amino acids.
Some of the reactions given by amino acids are as follows:
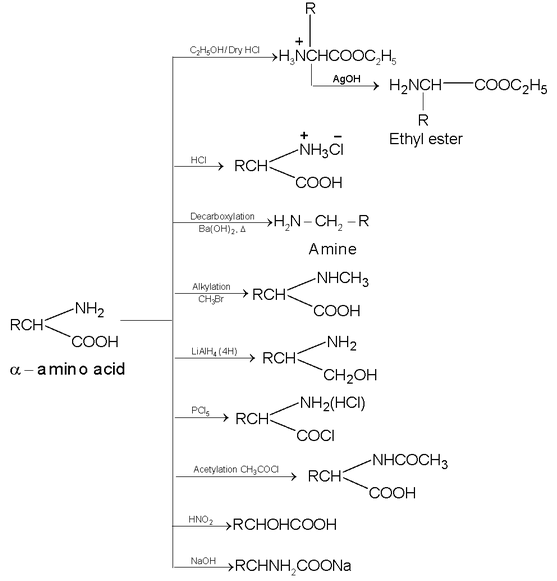
Action of heat:
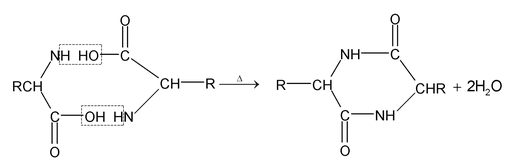
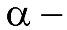 amino acids lose two molecules of water and form cyclic amides.
amino acids lose two molecules of water and form cyclic amides.
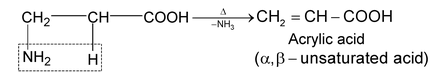
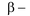 amino acids lose a molecule of ammonia per molecule of amino acid to yield
amino acids lose a molecule of ammonia per molecule of amino acid to yield
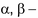 unsaturated acids.
unsaturated acids.