
Henry’s Law
Nov 02, 2022, 16:45 IST
This law was formulated at the beginning of the 19th century by the English chemist William Henry. It can be noted that Henry's constant can be expressed in two various ways. If the constant is defined in terms of solubility/pressure, it is referred to as Henry's law solubility constant that is denoted by "H." In contrast, if the proportionality constant is defined in terms of pressure/solubility, then it is called Henry's law volatility constant that is denoted by "k H ."
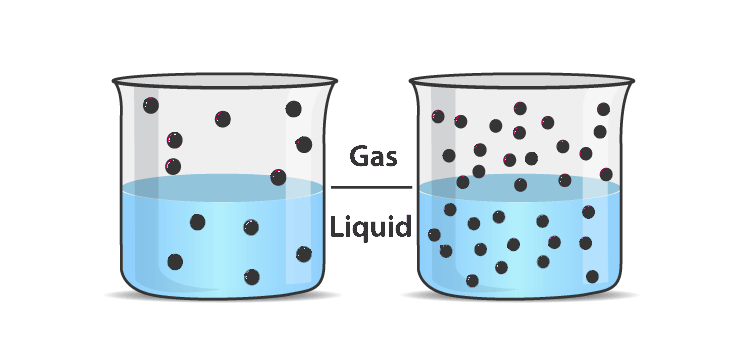
A relationship between the solubility of a gas in the given liquid and the partial pressure of that gas in the atmosphere, which is above the liquid, is mentioned above. Always note that the greater the partial pressure of the gas, the greater its solubility in the liquid.
| Table of Content |
What is Henry’s Law?
Henry's law explains the solubility of an gas in a liquid solution by the partial pressure and the mole fraction of the gas in the liquid. The formula of Henry’s law is:
P ∝ C (or) P = k H .C
Where,
P = Partial pressure of the gas in the atmosphere above the liquid
C = concentration of the dissolved gas
k H = Henry’s law constant of the gas
When Pressure is Constant?
When the pressure remains constant, according to henry’s law henry constant will be inversely proportional to the mole fraction of the gas. It can be represented as follows –
P ∝ C (Henry’s Law)
P = k H C
If P= constant, then the above equation can be written as –
C ∝ 1kH
It means if decrease in the value of Henry's law constant, the value of the mole fraction of gas in liquid will increases. Mole fraction of the gas in liquid is taken as solubility. Therefore, as henry’s law constant decreases, the solubility of the solute in solution increases.
Relation of Henry’s Law Constant with Temperature
We know that, on increasing the temperature than, the solubility of the gas in liquid decreases. So, we can write a relation between temperature and solubility for gas in liquid solution as follows –
T ∝ 1/ Solubility ----(1)
According to henry’s law, if the pressure is constant, as the value of henry’s law constant will increase, the solubility of a gas in liquid decreases. It can also be expressed as follow –
kH ∝ 1/Solubility - - -- (2)
From the given equations (1) and (2), we can write –
T ∝ kH
Factors affecting Henry’s Law Constant
The following factors that affect Henry’s Law of Constant of a Gas are–
- Nature of the solvent
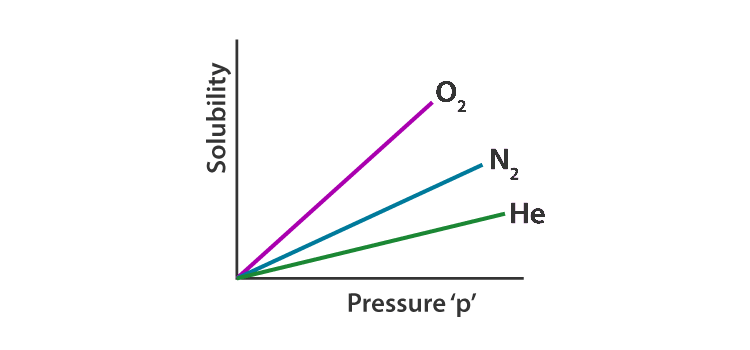
- Nature of the gas. This is why different gases have different henry’s law constants in the solvent.
- Temperature and Pressure of the system. This is why the k H value is given differently for gas at different temperatures.
Applications of Henry's Law
- The production of carbonated drinks is based on Henry's Law.
- Henry's law can explain hypoxia (low oxygen concentration in the blood and tissues).
- It is also applied during deep diving.
- It helps scuba divers breathe in high-pressure areas when air is diluted with He.
- In soft drinks increases the solubility of CO 2 and add flavor to the drinks.
- In areas where the partial pressure of O 2 is lower, the dissolution of O 2 during respiration helps.
Limitations of Henry's Law
- Henry's law is only applicable when molecules of a system are in the state of equilibrium.
- Henry's law is not applicable when the gas and the solution participate in chemical reactions with each other.
- This law does not hold when gases are placed under extremely high pressure.
Solved Examples
Q1. Find the solubility of gaseous oxygen in water at a temperature of 293 K when the partial pressure exerted by O 2 is 1 bar. (Given: kH for O 2 34840 bar.L.mol -1 )
Ans. we know P = k H .C
Substituting, k H = 34840 bar.L.mol -1 and P = 1 bar, the equation becomes
C = 1/34840 mol.L -1 = 2.87 x 10-5 mol/L
Thus, the solubility of oxygen in water under the given conditions is 2.87 x 10 -5 M.
Q2. The value of kH for carbon dioxide at a temperature of 293 K is 1.6 x 10 3 atm.L.mol -1 . At what partial pressure would the gas have a solubility (in water) of 2 x 10 -5 M?
Ans. Substituting the given values kH = 1.6 x 10 3 atm.L.mol -1 and C = 2 x 10 -5 M into the Henry’s law formula:
P = k H .C = (1.6 x10 3 atm.L.mol -1 ) x (2 x 10 -5 mol.L -1 ) = 0.032 atm.
Frequently Asked Question (FAQs)
Q1. Write the unit for Henry’s law constant?
Ans. Mol L –1 bar –1
Q2. Why Henry's law is not applicable at high pressure?
Ans. Henry's law is only applicable when the molecules are in the state of equilibrium. It does not apply to gases at high pressures (for example, N2(g) at high pressure becomes very soluble and dangerous when introduced into the blood supply).
Q3. Which gases do not obey Henry's law?
Ans. The dissolution of an ammonia gas in water do not obey Henry's Law.
Q4. What does Henry's law constant depends on?
Ans. It is important to note that Henry's law constants are highly dependent on temperature, since both vapor pressure and solubility are also temperature dependent.
Q5. Does Henry's constant depends on temperature?
Ans. Value of Henry's constant, K H increases with increase in temperature.









