
Hybridization
Sep 02, 2022, 16:45 IST
In chemistry, hybridization is defined as the concept of mixing two atomic orbitals to form a new type of hybridized orbitals. This mixing usually results in hybrid orbitals with completely different energies, shapes, etc. Hybridization mainly involves atomic orbitals of the same energy level. However, filled and half-filled orbitals can participate in this process if they have the same energy.
Similarly, we can say that the concept of hybridization is an extension of the theory of valence bonds and helps us understand the formation of bonds, the energy of bonds, and their lengths.
| Table of Content |
Hybridization definition
A redistribution of the energy of individual atomic orbitals to give orbitals of equivalent energy occurs when the two atomic orbitals combine to form a hybrid orbital in a molecule. This process is known as hybridization. During the hybridization process, atomic orbitals of comparable energies are mixed. They mostly involve merging two 's' orbitals or two 'p' orbitals or mixing an 's' orbital with a 'p' orbital and an 's' orbital with a 'd' orbital. A new orbitals thus formed are known as hybrid orbitals. More importantly, hybrid orbitals are quite helpful in explaining the bonding properties of atoms and molecular geometry.
Key Features of Hybridization
- Atomic orbitals with equal energies undergo hybridization.
- The no. of hybrid orbitals formed equals the number of mixed atomic orbitals.
- Not all half-filled orbitals need to participate in hybridization. Filled orbitals with slightly different energies can also participate.
- Hybridization occurs only during bond formation and not in an isolated gas atom.
- The shape of a molecule can be determined if the hybridization of the molecule is known.
- The larger lobe of the hybrid orbital always has a positive sign, while the smaller lobe has a negative sign on the opposite side.
Types of Hybridization
The hybridization can be classified as sp 3 , sp 2 , sp, sp 3 d, sp 3 d 2 , and sp 3 d 3 . Let us now discuss the various types of hybridization, along with their examples.
sp Hybridization
sp hybridization is determined when one s and one p orbital are in the same main shell of an atom that is mixed to form two new equivalent orbitals. The new orbitals formed are known as sp hybridized orbitals. It forms a linear molecule at 180°.
- This type of hybridization involves mixing one ‘s’ orbital and one ‘p’ orbital of the same energy to give a new hybrid orbital called sp hybridized orbital.
- Each sp hybridized orbital has an equal amount of s and p character, i.e., 50% p character and 50% s character.
- sp hybridization is also known as diagonal hybridization.
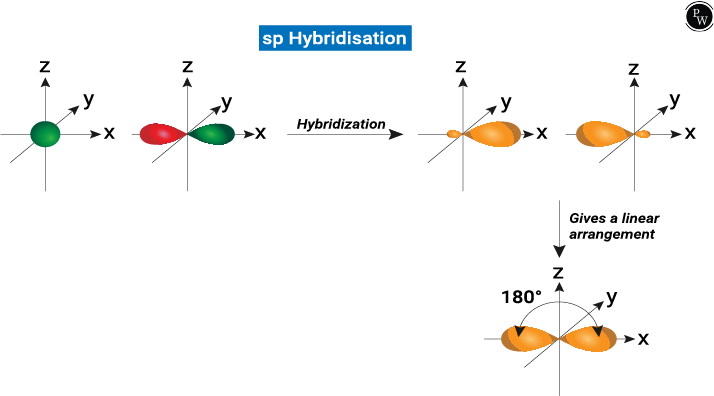
Examples of sp Hybridization:
- All compounds of carbon-containing triple bond like C 2 H 2 .
- All compounds of beryllium like BeF 2 , BeH 2 , BeC l2
sp 2 hybridization
sp 2 hybridization is determined when 1 s and 2 p orbitals of the equal shell of an atom are mixed to form 3 equivalent orbitals. The new orbitals formed are known as sp 2 hybrid orbitals.
- sp 2 hybridization is also known as trigonal hybridization.
- It combines 1 ‘s’ orbital and 2 ‘p’ orbital’s of the same energy to give a new hybrid orbital called sp 2 .
- s and p orbital mixture form trigonal symmetry and are maintained at 1200.
- All the 3 hybrid orbitals remain in 1 plane and make a 120° angle with one another. Each hybrid orbital formed has a 66.66% ‘p’ character and 33.33% ‘s’ character.
- The central atom of the molecule is linked to three atoms and is sp 2 hybridized have a triangular planar shape.
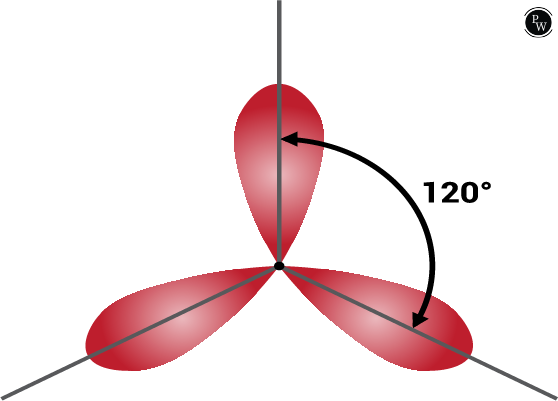
sp 3 Hybridization
When the 1 ‘s’ orbital and 3 ‘p’ orbitals belong to the same shell of an atom that is mixed together to form 4 new equivalent orbitals, this type of Hybridization is known as sp3 or tetrahedral Hybridization. The new orbitals formed are known as sp3 hybrid orbitals.
- These are directed towards the 4 corners of a regular tetrahedron and make a 109°28’ angle with one another.
- Each sp 3 hybrid orbital has 75% p character and 25% s character.
- An angle between the sp 3 hybrid orbitals is 109.280
- Example of sp3 Hybridization: ethane (C 2 H 6 ), methane.
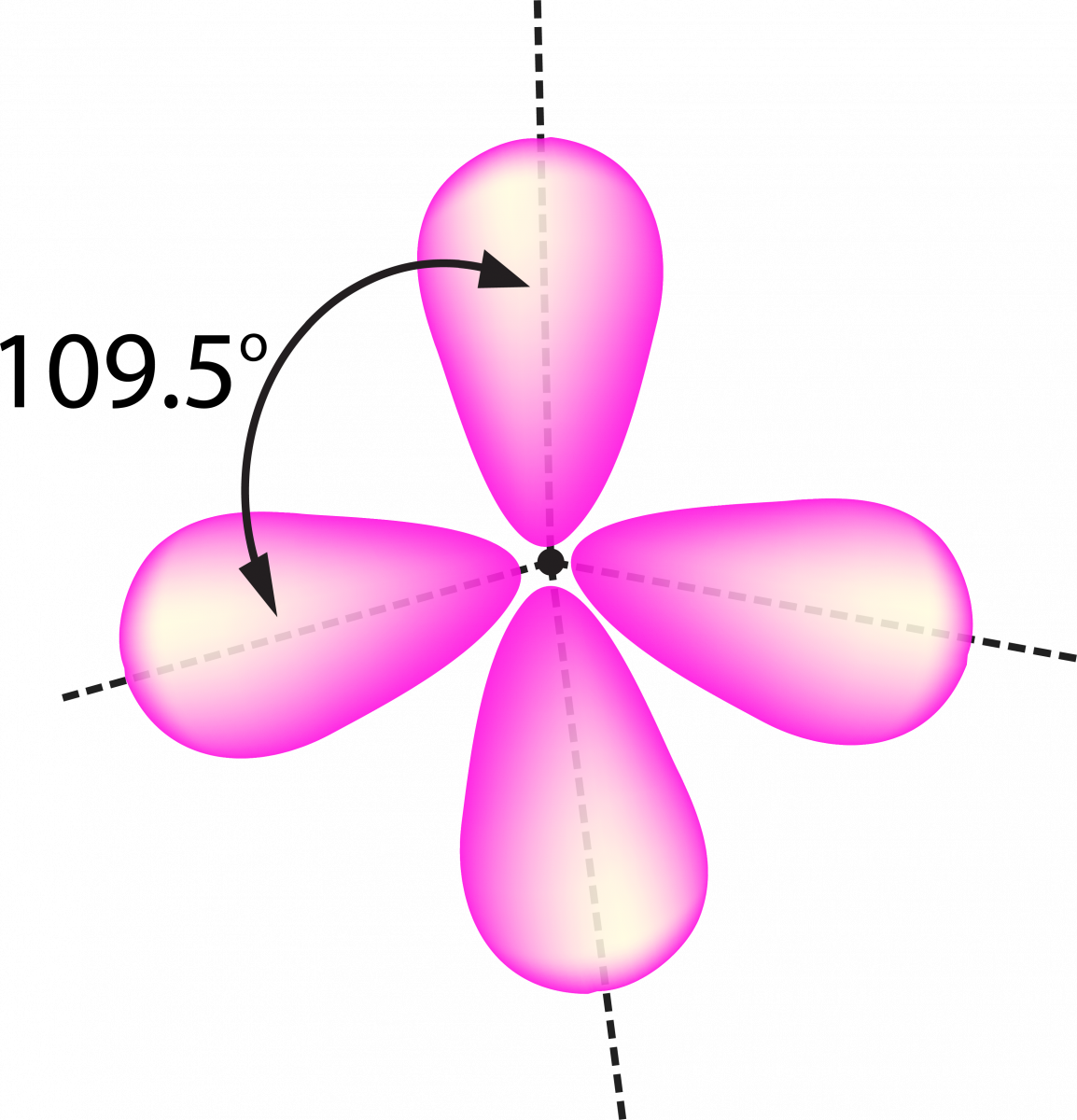
sp 3 d Hybridization
sp 3 d hybridization consists of mixing 1s orbital, 3p orbitals, and 1d orbital, usually forming five sp 3 d hybridized orbitals of the same energy. They have trigonal bipyramidal geometry.
- 3 hybrid orbitals usually lie in the horizontal plane inclined at an angle of 120° to each other, known as the equatorial orbitals.
- The s, p, and d orbital mixture form trigonal bipyramidal symmetry.
- The remaining 2 orbitals lie in the vertical plane at an angle 90 degrees plane of the equatorial orbitals, known as axial orbitals.
- Example: Hybridization in Phosphorus pentachloride (PCl 5 )
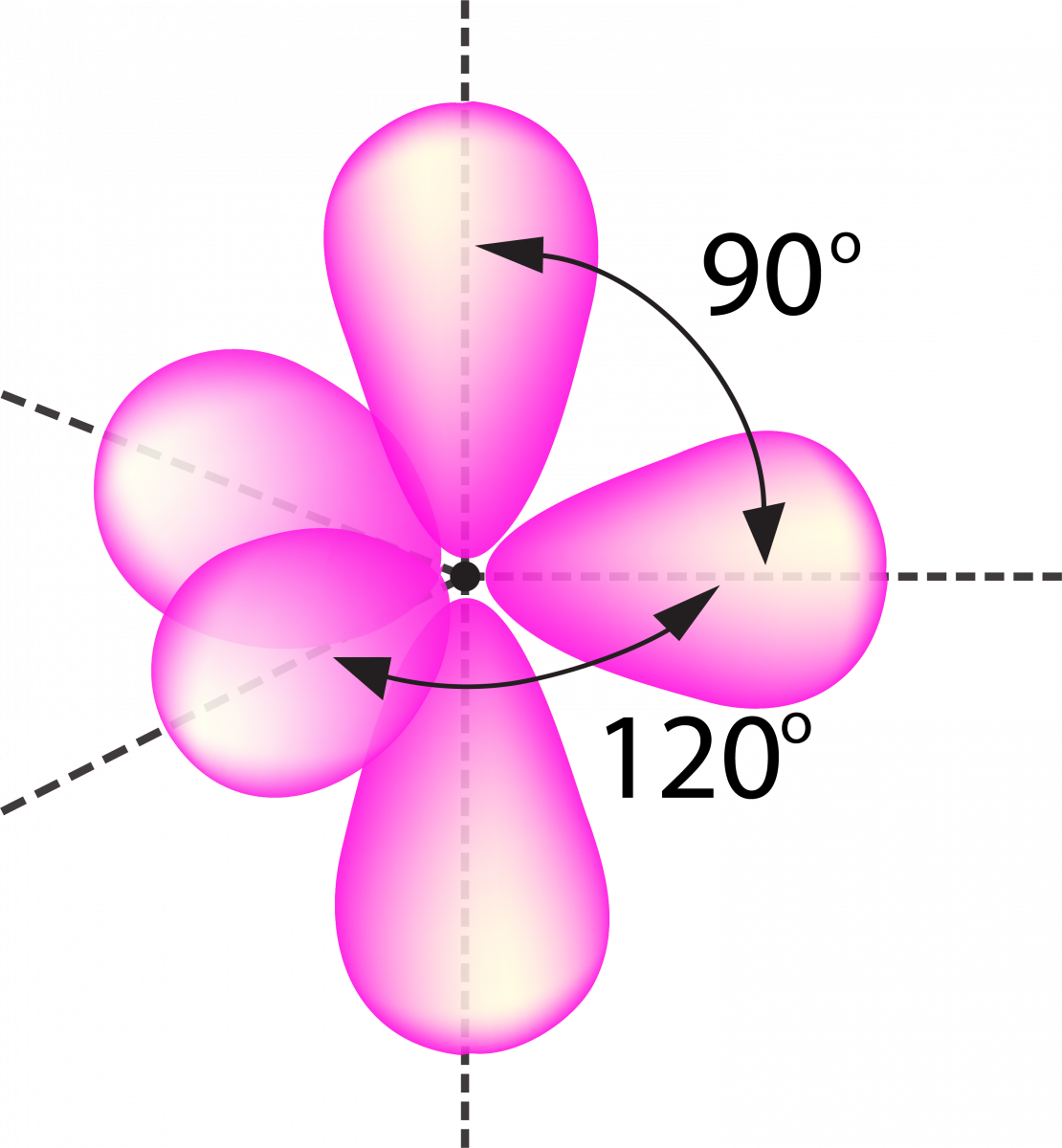
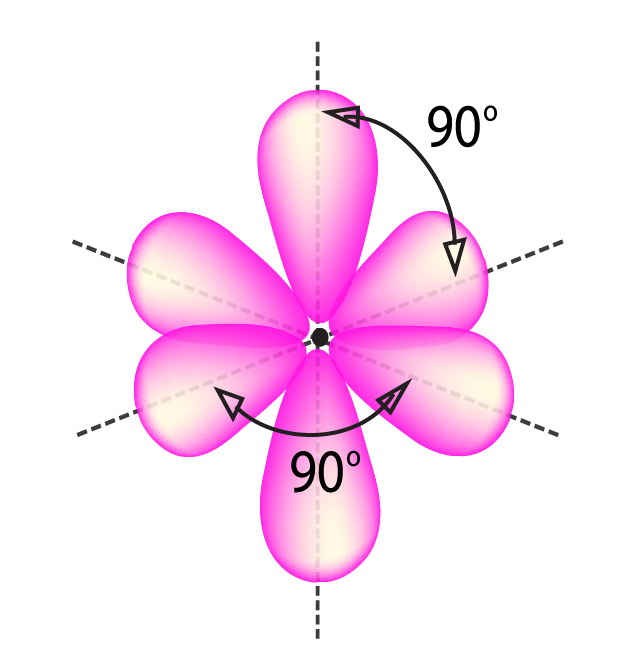
sp 3 d 2 Hybridization
- It has 1s, 3p, and 2d orbitals that undergo intermixing to form six identical sp 3 d 2 hybrid orbitals.
- These six orbitals are attracted to the corners of an octahedron.
- They are inclined at 90 degrees angle to one another.
Frequently Asked Question (FAQs)
Q1. What is Hybridization?
Ans. It is defined as the concept of mixing the two atomic orbitals to give rise to a new type of hybridized orbitals. This intermixing generally results in the formation of hybrid orbitals having entirely different energies, shapes, etc.
Q2. Is hybridization exothermic?
Ans. Hybridization is an exothermic reaction. Say, for sp3 hybridization. You have your s orbital and 3 p orbitals. To create four sp3 orbitals, you must promote your electron in the s-orbital to an empty p orbital.
Q3. What are the different types of hybridization?
Ans. The hybridization can be classified as follows,
- sp hybridization (beryllium chloride, acetylene)
- sp 2 hybridization (boron trichloride, ethylene)
- sp 3 hybridization (methane, ethane)
- sp 3 d hybridization (phosphorus pentachloride)
- sp 3 d 2 hybridization (sulphur hexafluoride)
- sp 3 d 3 hybridization (iodine heptafluoride)
Q4. Does hybridization require energy?
Ans. Hybridization requires energy because both promotion and hybridization require an input of energy, the formation of a set of singly occupied hybrid atomic orbitals is energetically uphill.
Q5. What is the purpose of hybridization?
Ans. Hybridization helps to explain molecule shape since the angles between bonds are approximately equal to the angles between hybrid orbitals.









