
What are the characteristics of long form of periodic table?
Aug 26, 2022, 16:45 IST
General Characteristics of Long form Periodic Table:
1. The 18 vertical columns of long form of periodic table, are called groups.
2. Some groups have typical names.
a. Elements of group 1 are called alkali metals.
b. Elements of group 2 are called alkaline earth metals
c. Elements of group 15 are called pnicogens
d. Elements of group 16 are called chalcogens
e. Elements of group 17 are called halogens
f. Elements of group 18 are called noble gases.
g. Elements of group 11 are called coinage metals.
3. The 7 horizontal rows or columns of long form of periodic table are called periods. i.e.,
a) First period : It contains two elements i.e., H & He. It is called shortest period
b) Second period : It contains eight elements e. Li to Ne. It is called short period. The elements of second period are also known as bridge elements.
c) Third period :It also contains 8 elements i.e. Na to Ar and known as short period. The elements of this period are also known as typical elements.
d) Fourth period : It contains 18 elements, K to Kr and this is called long period.
e) Fifth period : It has also 18 elements, Rb to Xe. This is called long period.
f) Sixth period : It has 32 elements, Cs to Kr. This period is a longest Period.
g) Seventh period : It is an incomplete period which has 29 – elements, Fr to Uuo.
4. The elements of group 1, 2 & 13 to 17 are called main group elements. These are also called as representative or normal elements.
5. The element of group 3 to 12 are called transition elements.
6. Elements with atomic number 58 to 71 are called lanthanides whereas the elements with atomic number 90 to 103 are called actinides. These elements are also known as f-block elements or inner-transition elements.
7. Lanthanides (4f-series) and actinides (5f-series) are placed in two separate rows below the main periodic table to avoid unnecessary side wise expansion of the periodic table.
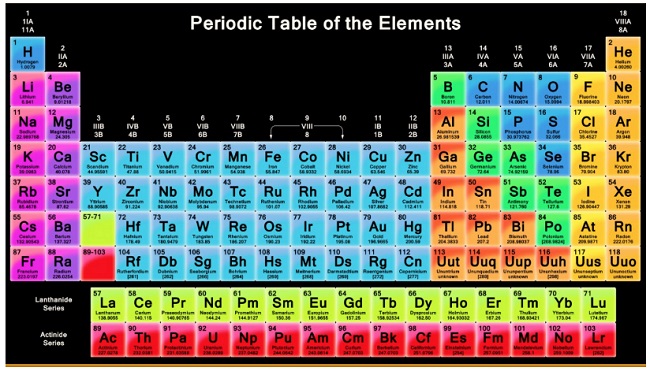
Table : Period and Energy Levels in Periodic Table
Physics Wallah Chemistry Doubts page consist of more questions for reference.
|
Periods |
Energy Level |
No.of elements |
Common name |
|
1 st |
1s |
2 (H to He) |
Shortest Period |
|
2 nd |
2s, 2p |
8 (Li to Ne) |
Short Period |
|
3 rd |
3s, 3p |
8 (Na to Ar) |
|
|
4 th |
4s 3d 4p |
18 ( K to Kr) |
Long period |
|
5 th |
5s, 4d 5p |
18 (Rb to Xe) |
|
|
6 th |
6s 4f 5d 6p |
32 (Cs to Rn) |
longest period |
|
7 th |
7s 5f 6d 7p |
29 |
Incomplete, it can accommodate upto 32 elements |
Advantage of Long form of Periodic Table:
1. It is based on more fundamental property i.e. atomic number and electronic configuration.
2. It completely separates metals and non – metals.
3. Due to separation of two sub groups, dissimilar elements do not fall together.
4. It correlates the position of elements with their electronic configuration clearly.
5. The completion of each period is more logical.
6. Periodicity in properties can be easily visualized.
7. The greatest advantage of this periodic table is that this can be divided into four blocks namely s,p,d and f-blocks elements.
8. This arrangement of elements is easy to remember and reproduce.
9. It removes the anomalies of Mandeleev’s periodic table i.e., K 39 precides Ar 40 .
Defects of Long form of Periodic Table:
1. Position of Hydrogen is not fixed till now
2. Position of helium is not fully justified as its EC justifies it to be included in the s – block.
3. Position of lanthanides and actinides has not been given inside the table.
4. It does not reflect the exact distribution of electrons among all the orbitals of the atoms of all the element.
What are the characteristics of long form of periodic table? pdf









