
Gaseous States of matter include solid, liquid, gas, and plasma. They're distinguished by particle arrangement, energy, volume, and shape. Temperature and pressure influence transitions between states. Gaseous properties can be explained by various laws and gas equations.
Boyle's Law
Describes the inverse relationship between pressure (P) and volume (V) for a gas at constant temperature. Formula:P 1 × V 1 = P 2 × V 2
Charle's Law
States that the volume (V) of a gas is directly proportional to its absolute temperature (T) at constant pressure. Formula: T α V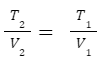
Gay-Lussac's Law
Describes the direct relationship between pressure (P) and absolute temperature (T) for a gas at constant volume. Formula: P α T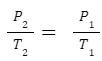
Avogadro's Law
States that the volume (V) of a gas is directly proportional to the number of moles (n) of the gas at constant temperature and pressure. Formula:Also Read – List of Chemistry Formulas
Ideal Gas Equation
This equation relates the pressure, volume, temperature, and amount of gas in a system. It assumes gases behave ideally under many conditions, although deviations can occur at very high pressures or low temperatures. Formula: PV=nRT Where: P = Pressure of the gas V = Volume occupied by the gas n = Number of moles of the gas R = Universal gas constant, which has a value of 8.314 J/(mol·K ) when the pressure is in pascals (Pa) and volume is in cubic meters (m³). If pressure is in atmospheres (atm) and volume is in litres (L) , R is 0.0821 L·atm/(mol·K). T = Absolute temperature in Kelvin (K) This equation is derived from the combination of Boyle's, Charles's, Gay-Lussac's, and Avogadro's laws. If you want to express the ideal gas equation in terms of density (ρ) and molar mass (M), you can use the following relationship:Also Check – Surface Chemistry Formula
Dalton's Law of Partial Pressures
In a mixture of non-reacting gases, the total pressure exerted by the mixture is equal to the sum of the partial pressures of the individual gases. Each gas in a mixture behaves as if it was the only gas present and occupies the entire volume. Formula: P total = P 1 + P 2 + P 3 +... Where: P total = Total pressure of the gas mixture. P 1 , P 2 , P 3 , ... = Partial pressures of the individual gases in the mixture. Using Dalton's Law of Partial Pressures, the partial pressure of a gas in a mixture can be related to its mole fraction. Formula: Pi = i × PT Where: P i = Partial pressure of gas i. χ i = Mole fraction of gas i. P total = Total pressure of the gas mixture. So, the partial pressure of a component in a gas mixture is equal to its mole fraction multiplied by the total pressure of the mixture. When dealing with gas mixtures, the concept of mole fraction is often used to describe the proportion of a particular gas in the mixture. The ratio of the number of moles of that gas to the total number of moles of all gases in the mixture is mole fraction.Download PDF Gaseous State Formula
Mole Fraction (χ)
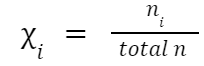 Where: χ
i
= Mole fraction of gas i.
n
i
= Moles of gas i.
total n = Total moles of all gases in the mixture.
Where: χ
i
= Mole fraction of gas i.
n
i
= Moles of gas i.
total n = Total moles of all gases in the mixture.
Kinetic Theory of Gases
Gases are small, constant particles in random motion, with no kinetic energy loss. Collisions between particles and containers are elastic, and the average kinetic energy is directly proportional to the gas's temperature. This relationship relates the molecular speed of gas particles to the temperature and molar mass of the gas.Root Mean Square Speed (u rms ): This is a measure of the average speed of particles in a gas.
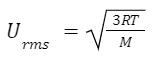 Where: R is the universal gas constant (in
J/(mol·K
) when working with SI units). T is the absolute temperature (in
Kelvin
). M is the molar mass of the gas (in
kg/mol
for SI units). Average Speed (u
avg
): This is another measure, slightly less than u
rms
, but closely related. Most Probable Speed (u
mp
): This speed is the speed at which the maximum number of particles is moving.
Where: R is the universal gas constant (in
J/(mol·K
) when working with SI units). T is the absolute temperature (in
Kelvin
). M is the molar mass of the gas (in
kg/mol
for SI units). Average Speed (u
avg
): This is another measure, slightly less than u
rms
, but closely related. Most Probable Speed (u
mp
): This speed is the speed at which the maximum number of particles is moving.
Van Der Waals Equation
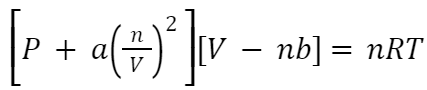 Where:
P is the pressure of the gas.
V is the volume of the gas.
n is the number of moles of the gas.
R is the universal gas constant.
T is the absolute temperature.
Where:
P is the pressure of the gas.
V is the volume of the gas.
n is the number of moles of the gas.
R is the universal gas constant.
T is the absolute temperature.
a and b are the van der Waals constants, which are specific to each gas.
The term a(n/v) 2 corrects for the attractive forces between particles: a measures intermolecular attraction strength, with larger values indicating stronger interactions. nb represents gas particle volume, representing excluded volume not available to other molecules.Also Check – Aluminium Nitrate Formula
Compressibility Factor (Z)
The compressibility factor is a measure of how much a real gas deviates from the behavior of an ideal gas. For an ideal gas, Z is equal to 1. Deviations from this value indicate non-ideality. Formula :Viscosity
Viscosity is a measure of a fluid's resistance to shear or flow. It describes how "thick" or "sticky" a fluid is. While viscosity is typically associated with liquids, gases also have viscosity.Gaseous State Formula FAQs
Q1. What does Boyle's Law state?
Q2. Define Dalton's Law of Partial Pressures.
Q3. What is the ideal gas equation?
Q4. Why do real gases deviate from ideal behavior?
Q5. How does temperature affect gas viscosity?










