
Carbohydrates: The most prevalent biomolecules on Earth are carbohydrates. Annually, photosynthesis converts more than 100 billion tons of CO 2 and water into other plant-based materials. Most people consume sugar and starch (types of carbohydrates) as dietary staples. The oxidation of carbohydrates is the central energy-yielding pathway in most non-photosynthetic cells. The term "carbohydrate polymers," aka glycans, functions as structural and defense components in the connective tissue, bacterial and plant cell walls, and tissues of animals. Other carbohydrates polymers maintain skeletal joint lubrication and play a role in recognizing and adhesion between cells.
Overview
Carbohydrates are polyhydroxy aldehydes, or ketones, or substances that yield such compounds on hydrolysis. Carbohydrates are also called sugars in general, some partially methylated sugars, amino sugars, and amino sugars naturally exist, and one natural nitro sugar is known.
Carbohydrates General Formula
The empirical formula (CH 2 O)n is shared by many, but not all, carbohydrates, some also contain nitrogen, phosphorus, or sulfur. Only simple sugars, which contain the same amount of carbon and water, can be used with this formula, aka "Hydrates of Carbon.". In recent years, carbohydrate structures rather than their formulas have been used to categorize different types of carbohydrates. We now refer to these aldehydes and ketones as polyhydroxy. Among the substances that fall into this category are cellulose, starch, and glycogen.
Carbohydrates and Its Classification
Carbohydrates come in two main categories: simple and complex . The quantity of sugar molecules that each possesses is what makes them different from one another. While complex carbohydrates include three or more sugar molecules, simple carbohydrates, usually referred to as simple sugars, only have one or two.
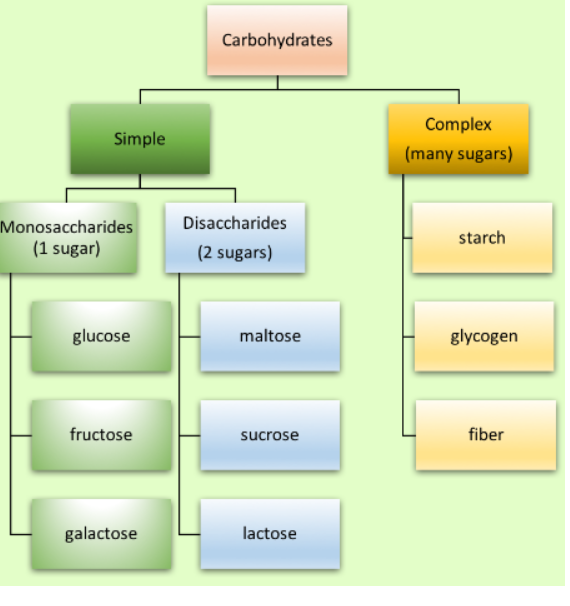
Monosaccharides, oligosaccharides, and polysaccharide s are the three main classes of carbohydrates (the word "saccharide" is derived from the Greek word “sakcharon," meaning "sugar").
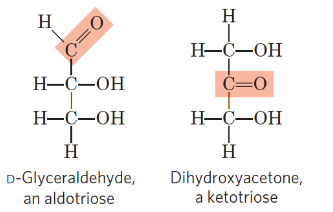
- Monosaccharides: or simple sugars, consist of a single polyhydroxy aldehyde or ketone unit. The most abundant monosaccharide in nature is the six-carbon sugar D-glucose, sometimes referred to as dextrose. Monosaccharides are crystalline, colorless solids that can dissolve freely in water but not in nonpolar solvents. Most of them taste sweet. Common monosaccharides have unbranched carbon chains as their backbones, in which all of the carbon atoms are connected by single bonds. One of the carbon atoms in this open-chain form forms a carbonyl group through a double bond with an oxygen atom, while each of the other carbon atoms has a hydroxyl group. The monosaccharide is an aldose if the carbonyl group is at the end of the carbon chain (i.e., in an aldehyde group), and it is a ketose if the carbonyl group is anywhere else (i.e., in a ketone group).The two three-carbon trioses - glyceraldehyde, an aldotriose , and dihydroxyacetone, a ketotriose , are the two simplest monosaccharides.
Tetroses, pentoses, hexoses, and heptoses are the names of the four, five, six, and seven carbon atoms that make up a monosaccharide's backbone, respectively. Each of these chain lengths has an associated set of aldoses and ketoses, such as aldotetroses and ketotetrose, aldopentoses and ketopentoses, and so on. The most prevalent monosaccharides in nature are the hexoses, which include aldohexose D-glucose and ketohexose D-fructose. They are byproducts of photosynthesis and important intermediates in the central energy-yielding reaction that produces energy in most organisms.
2. Oligosaccharides: consist of short chains of monosaccharide units, or residues, joined by characteristic linkages called glycosidic bonds. Disaccharides, which contain two monosaccharide units, are the most prevalent. Typical is sucrose (cane sugar), which is made up of D-glucose and D-fructose, two six-carbon sugars.
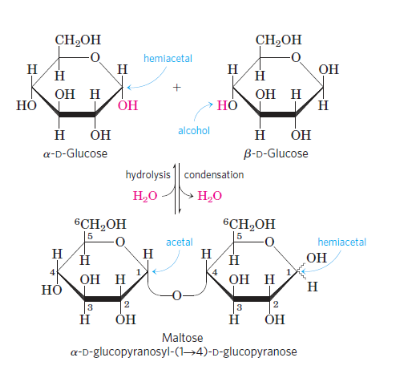
- Disaccharides (such as maltose, lactose, and sucrose) consist of two monosaccharides joined covalently by an O-glycosidic bond, which is formed when the hydroxyl group of one sugar molecule, typically cyclic, reacts with the anomeric carbon of the other.
- Two D-glucose residues make up the disaccharide maltose, which is connected by a glycosidic bond between the C-1 (the anomeric carbon) of one glucose residue and the C-4 of the other.Because the disaccharide retains a free anomeric carbon (C-1 of the glucose residue on the right in Fig. 7–10), maltose is a reducing sugar.
- Polysaccharides: The sugar polymers known as polysaccharides, contains more than 20 or so monosaccharide units; some have hundreds or thousands of units. Some polysaccharides, like cellulose, are made up of linear chains, while others, like glycogen, have branched chains. Both glycogen and cellulose are made of recurrent units of D-glucose, but they differ in the kind of glycosidic bond they have, and as a result, their characteristics and biological functions are very different.
The majority of carbohydrates that are found in nature are polysaccharides, which are polymers with molecular weights ranging from 20,000 to more. Polysaccharides, also called glycans, differ from each other in the identity of their recurring monosaccharide units, in the length of their chains, in the types of bonds linking the units, and in the degree of branching.
Homopolysaccharides: contain only a single monomeric species Some homopolysaccharides, such as starch and glycogen, act as storage molecules for monosaccharides that are used as fuels. Other homopolysaccharides (Chitin and cellulose, for instance) act as structural components of animal exoskeletons and plant cell walls.
Heteropolysaccharides: Heteropolysaccharides provide extracellular support for organisms of all kingdoms. For example, the rigid layer of the bacterial cell envelope (the peptidoglycan) is composed, in part, of a heteropolysaccharide built from two alternating monosaccharide units.










