
Molecular Orbital Theory (MOT)
- Molecular Orbital Theory was proposed by Hund & Mulliken.
- With the help of MOT, we can explain and understand those things that VBT was unable to explain. (Exp. Paramagnetic nature of O 2 molecule. As per VBT, it should be Diamagnetic.)
- The atomic orbital loses its identity during molecule formation (by overlapping) and forms new orbitals called molecular orbitals.
-
Molecular orbital formed by overlapping of atomic orbital of same energy.
-
Electrons in a molecule occupy molecular orbitals in accordance with Aufbau principle, Pauli’s exclusion principle and Hund’s rule.
-
The number of molecular orbitals formed = Number of atomic orbitals involved in overlapping.
-
The shape of a molecular orbital is the region around the nuclei where there exists 90–95% probability of finding the electron.
-
There are two types of molecular orbitals: Bonding Molecular Orbitals (BMO) and Antibonding Molecular Orbitals (ABMO).
-
Half of the molecular orbital which have lower energy, called Bonding molecular orbital (BMO).
-
Half are of higher energy - termed as Antibonding molecular orbital (ABMO).
-
Wave function for bonding molecular orbital is
-
Wave function for antibonding molecular orbital is
Linear Combination of Atomic Orbital (LCAO)
Linear Combination of Atomic Orbital (LCAO) : The effective combinations of atomic orbitals of atoms A and B are as follows:
1. Combination Involving 2 s (A) and 2 s (B)
2. Combination Involving 2P z (A) and 2P z (B)
When two orbitals combine in the same phase then constructive interference take place. When two orbitals combine out of the phase then destructive interference takes place.
3. Combination Involving 2p x (A) and 2p x (B)
4. Combination Involving 2p y (A) and 2p y (B)
The molecular orbitals formed are similar to π2p x and π*2p x in the direction of y – axis.
Electron Density in BMO :
Electron Density in ABMO :
Difference Between BMO And ABMO :
|
Bonding Molecular Orbital (BMO) |
Antibonding Molecular Orbital (ABMO) |
|
| 1. | MO formed by the addition of atomic orbitals | MO formed by the subtraction of atomic orbitals |
| 2. |
|
|
| 3. | Formed by constructive. Interference (Stabilized MO) | Formed by destructive interference. (Destabilized MO) |
| 4. | Lower in energy as compared to atomic orbital | Higher in energy as compared to atomic orbital |
| 5. | Electron density increases in the internuclear region | Electron density decreases in the internuclear region |
| 6. | May or may not have a nodal plane | Always has a nodal plane |
| 7. | Represented by σ1s, σ2pz, π2p x , π2p y | Represented by σ*1s, σ*2pz, π*2p x , π*2p y |
Shape of the Molecular Orbital :
Shapes of MOs Formed by s-orbitals : When two orbitals combine in same phase then constructive interference takes place.
When two orbitals combine out of the phase then destructive interference takes place.
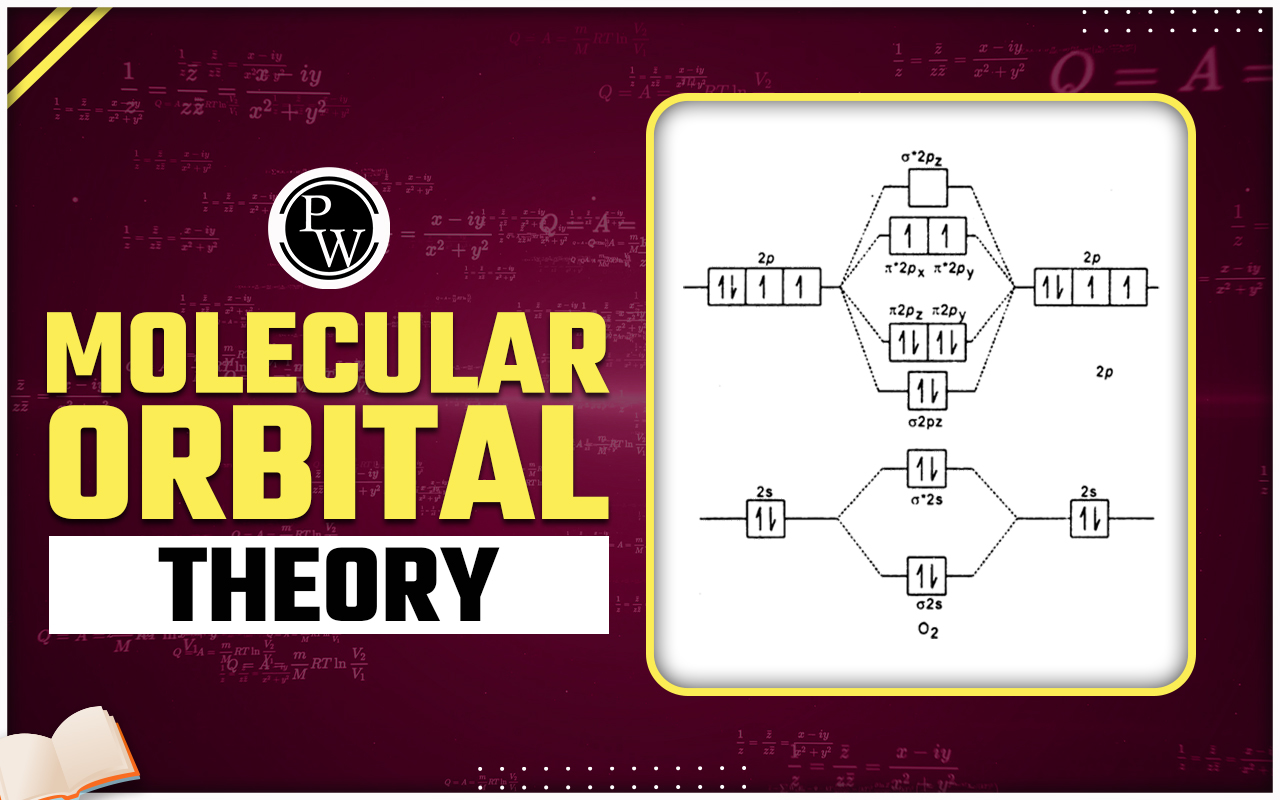
Molecular Orbital Energy Diagram
Electronic Configuration of Molecular Orbitals
Note : Molecular orbital energy diagram for up to N 2 (molecule having ≤ 14 electrons)
Note : Molecular orbital energy diagram for O 2 and F 2 (molecule having > 14 electrons)
= Antibonding molecular orbital
σ, π = Bonding molecular orbital
Shapes of Molecular Orbital 𝞂 2P z :
Applications of Molecular Orbital Theory (MOT)
Bond order =
N b = No. of electron in bonding MO’s
N a = No. of electron in antibonding MO’s
-
If bond order = 0, it means species does not exist.
-
Bond order of 1, 2 & 3 corresponds to a single bond, double & triple bond respectively.
-
Bond order ↑ stability of molecules ↑ bond length ↓
Magnetic behaviour
-
If the molecule has one or more unpaired electron, it will be paramagnetic,
-
If all the electrons paired it will be diamagnetic.
-
Magnetic strength can be calculated by using spin only formula of magnetic moment (μ).
-
μ =
B. M. (where n = number of unpaired electron)
Ex.
H
2
= Configuration:
Bond order =
,
Hence H – H (diamagnetic)
| S.N. |
Molecules/ ions |
Molecular Orbital (MO) Configuration |
Bond Order |
Magnetic Character |
|
1. |
H 2 |
σ(1s) 2 |
1 |
Diamagnetic |
|
2. |
|
σ(1s) 1 |
|
Paramagnetic |
|
3. |
C 2 |
…. σ(2s) 2 σ * (2s) 2 π(2p x ) 2 π(2p y ) 2 |
2 |
Diamagnetic |
|
4. |
O 2 |
…. σ(2s) 2 σ * (2s) 2 σ(2P z ) 2 π(2P x ) 2 = π(2P y ) 2 π(2P x ) 1 = π(2P y ) 1 |
2 |
Paramagnetic |
|
5. |
|
…. σ(2s) 2 σ * (2s) 2 σ(2P z ) 2 π(2P x ) 2 = π(2P y ) 2 π*(2P x ) 1 = π*(2P y ) 0 |
2.5 |
Paramagnetic |
|
6. |
CN |
…. σ(2s) 2 σ * (2s) 2 π(2P x ) 2 = π(2P y ) 2 σ(2P z ) 1 |
2.5 |
Paramagnetic |
|
7. |
NO + |
….. σ(2s) 2 σ * (2s) 2 π(2P x ) 2 = π(2P y ) 2 σ(2P z ) 2 |
3.0 |
Diamagnetic |
Molecular Orbital Theory FAQs
Q.1 : What is Molecular Orbital (MO) Theory?
Q.2 How does Molecular Orbital Theory (MOT) differ from Valence Bond Theory (VBT)?
Q.3 What is the significance of Molecular Orbital Theory in predicting molecular properties?
Q.4 What are molecular orbitals?
Q.5 Can Molecular Orbital Theory predict the magnetic properties of molecules?












