
Rutherford’s Model Of Atom : Ernest Rutherford's model of the atom, proposed in 1911, revolutionized the prevailing notion of atomic structure. Building upon J.J. Thomson's discovery of the electron and the plum pudding model, Rutherford introduced the concept of a dense, positively charged nucleus at the centre of the atom. According to this model, electrons orbit the nucleus in distinct energy levels, akin to planets orbiting the sun.
- The cornerstone of Rutherford's model lies in the famous gold foil experiment conducted by Rutherford and his colleagues Hans Geiger and Ernest Marsden. It is the continue of next line.
- In this experiment, A narrow beam of alpha particles (positively charged particles) was directed at a thin gold foil.
Rutherford’s Gold Foil Experiment
Rutherford’s Gold Foil Experiment Observations
The expected outcome, based on Thomson's plum pudding model, was that the alpha particles would pass through the foil with minimal deflection, as the positive charge was thought to be uniformly distributed throughout the atom.
- Most of the particles (99%) passes through it, without any deviation or deflection.
- Some of the particles were deflected through small angles.
- Very few particles were deflected by large angles, and occasionally, a particle got deflected by 180°
- Some were deflected at large angles, and a few even bounced back in the direction from which they came.
- This unexpected scattering pattern led Rutherford to conclude that the majority of an atom's mass and positive charge is concentrated in a tiny, dense nucleus at its centre.
- There must be a very heavy and positively charged body in the atom i.e. nucleus, which does not permit the passage of positively charged particles.
- Because the number of particles that undergo deflection of 180º is very small, therefore the volume of the positively charged body must be an extremely small fraction of the total volume of the atom. This positively charged body must be at the centre of the atom, which is called the nucleus.
- The deflection of alpha particles indicated that All the positive charge and mass of the atom is present in a very small region at the centre of the atom. It is called the nucleus.
- The nucleus is surrounded by electrons that move around the nucleus at a very high speed in circular paths called orbits.
- The centrifugal force is present due to fast-moving electrons balancing the coulombic force of attraction present between the nucleus and the electrons.
- Rutherford’s atomic model is comparable with the solar system. So it is known as planetary model .
The volume occupied by the nucleus is negligibly small as compared to the total volume of atom. The radius of the atom is about 10 –10 m, while that of nucleus is 10 –15 m.
Rutherford estimated the size of nucleus by calculating the distance of closes approach.
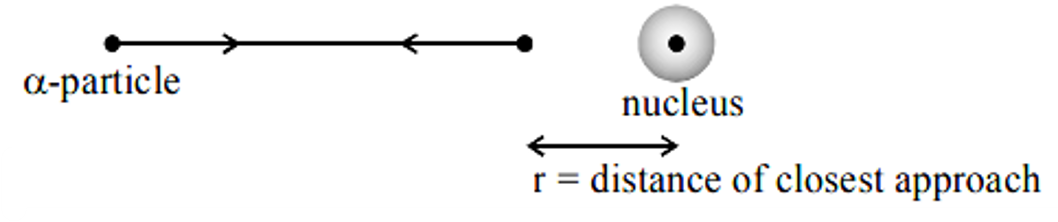 The initial kinetic energy of
The initial kinetic energy of
It fails to explain the stability of an atom: According to electromagnetic theory, when a charged particle moves with acceleration, they emits energy in the form of electromagnetic radiation.
According to Rutherford's model, electrons revolve around the nucleus and emit energy in the form of electromagnetic radiation, as a result of which electron loses energy continuously and should collapse on the nucleus following a spherical path. Rutherford's model failed to explain the stability of atoms.
- it could not explain the line spectrum of the H-atom.
- It says nothing about the electronic structure of atoms i.e. how the e – are distributed around the nucleus and what are the energies of these e – .
Important Points To Remember
- Thomson’s model of atom explained electrical neutrality of atom.
- Density of nucleus remains same irrespective of nature of element.
Rutherford’s Model Of Atom Examples
Ex.1
An
-particles of kinetic energy of 5.4 MeV is projected towards gold nucleus. Calculate the distance of closet approach. (Atomic number of gold = 79, 1 eV = 1.6 × 10
–19
J)
Sol:
Ex.2
An
-particles is projected towards the following nucleus with same kinetic energy in different experiment the distance of closet approach is maximum for:
(A) Na (Z = 11) (B) Ca (Z = 20)
(C) Ag (Z = 47) (D) Au (Z = 79)
Sol: (A)
Rutherford’s Model of Atom FAQs
Q.1 : What is Rutherford's Model of the Atom?
Q.2 : What was Rutherford's Scattering Experiment?
Q.3 : What were the Observations of Rutherford's Scattering Experiment?
Q.4 : How did Rutherford's Model explain the Observations?
Q.5 : What are the Drawbacks of Rutherford's Model?
Q.6 : How was Rutherford's Model Improved?
Q.7 : What are the Educational Implications of Rutherford's Model?








