Hypotonic Solution: Tonicity is the measurement of the effective osmotic pressure gradient. It involves comparing the water potential of two solutions separated by a partially permeable cell membrane. Tonicity is determined by the relative concentration of solutes that the membrane allows to pass through, which affects the extent and direction of osmotic flow.
A solution can be classified as hypotonic, hypertonic, or isotonic compared to another solution. A hypotonic solution has a lower solute concentration than the other solution across a semipermeable membrane. This causes water to move into the cell, leading to swelling or even bursting. As a result, the osmotic pressure in a hypotonic solution is lower than in other solutions. Information about hypertonic solutions in NEET Biology Notes can be found in the article below.Hypotonic Solution Definition
The capacity of an extracellular solution to influence water movement into or out of a cell via osmosis is termed 'tonicity'. Tonicity correlates with the solution's osmolarity. Examination of a hypotonic solution can enhance comprehension of this concept. Osmolarity denotes the collective concentration of solutes within a solution. Lower osmolarity signifies fewer solute particles per litre, while higher osmolarity indicates a greater number of solute particles per litre. A hypotonic solution has a lesser solute concentration compared to another solution. The classification of a solution as hypotonic, isotonic, or hypertonic depends on its comparison to another solution. Understanding tonicity and osmolarity is vital for cell description. Osmolarity, measuring solute concentration per litre across various solutions, elucidates the formation of water and solute gradients. For NEET exam preparation, refer to NEET Biology MCQ and NEET Previous Year Question papers related to this topic.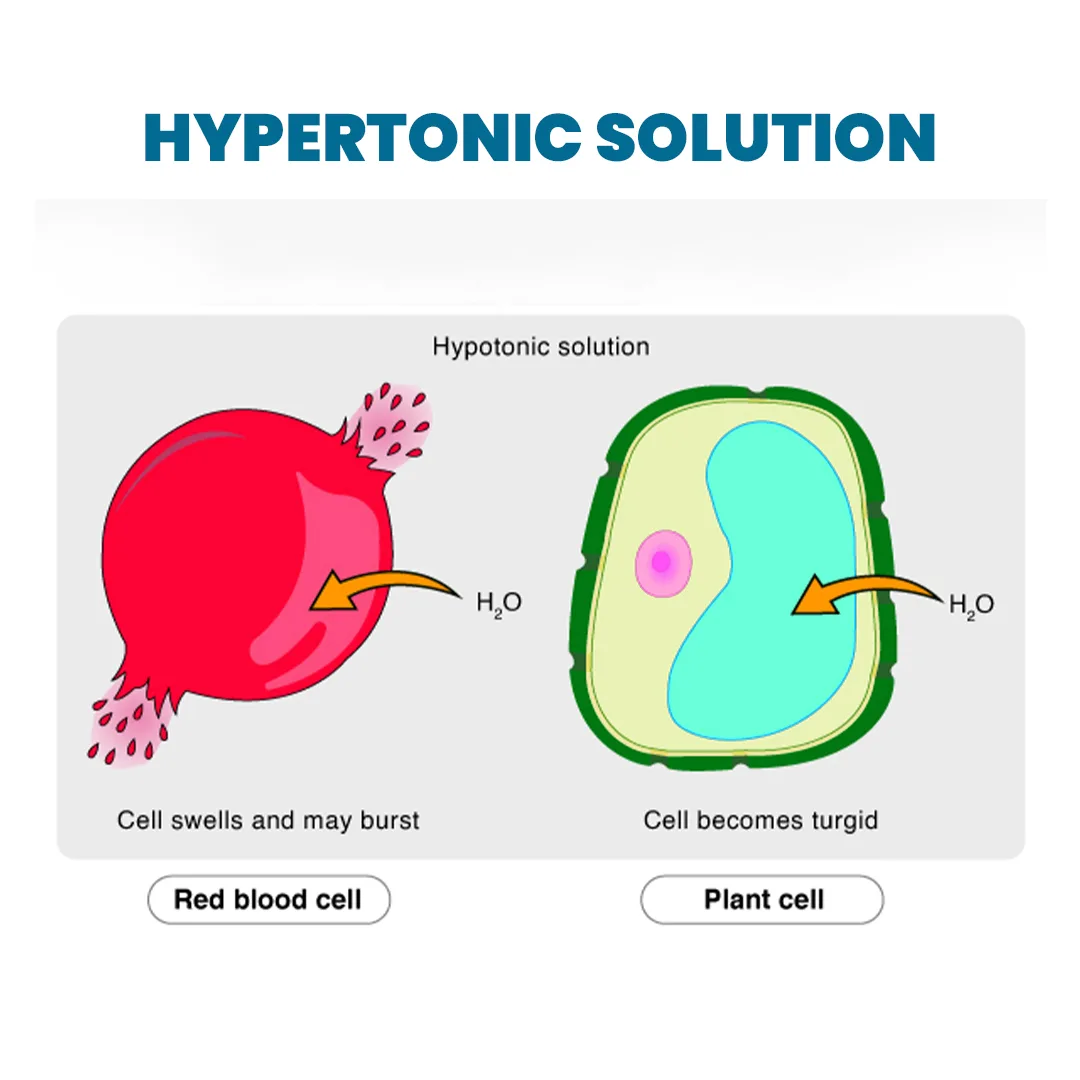
Hypotonic Solution Examples
Hypotonic solutions are those with a lower solute concentration compared to a reference solution, often a cell or blood plasma. This means that there is a higher proportion of water to solutes in a hypotonic solution. Here are some common hypotonic solution examples:- Distilled water: Distilled water has minimal solutes, making it hypotonic to most cells.
- Plain, unsweetened tea or coffee: These beverages have some dissolved substances, but much less than blood or cells.
- Coconut water: While it contains some electrolytes, coconut water is generally considered hypotonic to blood plasma.
- Certain sports drinks: Some sports drinks are designed to be hypotonic to assist in rehydration after exercise. However, it's important to check the label, as some sports drinks can be hypertonic.
Hypotonic Solution Swell or Shrink
Hypotonic solutions contain a lower concentration of solutes, such as salts and sugars, compared to the solute concentration inside the cell. This difference in concentration causes water to move into the cell through osmosis, leading to the cell swelling. Osmosis is the process of water moving through a semipermeable membrane from an area of low solute concentration (hypotonic solution) to an area of high solute concentration (the cell) to equalize concentrations on both sides. This movement of water can cause cells to shrink or swell depending on the surrounding solution. Visualize a cell as a small balloon filled with sugary water (representing cytoplasm) and surrounded by a thin, elastic membrane (plasma membrane). The membrane acts as a gatekeeper, allowing some substances to pass through while keeping others out. In a hypotonic solution, which is like submerging our balloon in a large tub of plain water, the solute concentration outside the cell is lower than inside the cell. This creates a situation where water, being the key player, moves as follows:- Osmosis Takes Place: Water naturally moves from an area of low solute concentration (outside the cell) to an area of high solute concentration (inside the cell) to balance the concentrations.
- Water Influx: Because there's more sugary water inside the cell than outside, water molecules flow into the cell through the semipermeable membrane.
- Cell Swells: As water enters, the cell swells and expands. The more water that enters, the larger the cell becomes.
Hypotonic Solution Importance
Hypotonic solutions, with their lower solute concentration compared to cellular environments, play a pivotal role in biological processes. These solutions facilitate water influx into cells through osmosis, serving several key functions:- Hydration: Hypotonic solutions are crucial for rehydrating individuals experiencing dehydration from conditions such as diarrhoea, vomiting, or burns. The influx of water into cells restores proper cellular function.
- Drug Delivery: These solutions are utilised to deliver medications or nutrients directly into cells, aided by osmotic pressure gradients.
- Cellular Swelling: Controlled cell swelling can be beneficial in certain medical conditions. For instance, hypotonic solutions are used to treat glaucoma by increasing fluid pressure within the eye, aiding in lens repositioning and improved drainage.
- Food Preservation: They are used to shrink bacterial cells, prolonging the shelf life of food products.
- Agriculture: Hypotonic solutions promote seed germination by facilitating water uptake, crucial for initial plant growth stages.
Maintaining Cellular Equilibrium
While hypotonic solutions offer various benefits, maintaining solute concentration balance is critical. Excessive water influx can cause cell swelling and potential bursting, while excessive efflux can lead to cell shrinkage and dehydration, both detrimental to cell health. In summary, hypotonic solutions are integral to biological processes and have significant medical and industrial applications. Understanding their impact on cells and the importance of cellular balance is crucial for their effective and safe use.Hypotonic Solution and Hypertonic Solution
Hypotonic and hypertonic solutions are important concepts in biology, particularly in understanding how cells interact with their environment. These solutions have different effects on cells due to their differing concentrations of solutes. Here's a comparison table to highlight the key differences:| Hypotonic Solution vs. Hypertonic Solution | ||
|---|---|---|
| Aspect | Hypotonic Solution | Hypertonic Solution |
| Definition | Lower concentration of solutes than the cell | Higher concentration of solutes than the cell |
| Effect on Cells | Causes cells to swell (cytolysis) | Causes cells to shrink (plasmolysis) |
| Osmotic Pressure | Lower than the cell | Higher than the cell |
| Example | Pure water | Saline solution |
| Biological Importance | Prevents cells from drying out; used in cell culture | Preserves food by drawing out water from bacteria |
| NEET Exam Important Links | |
|---|---|
| NEET Syllabus | NEET Biology Diagrams |
| NEET Biology Notes | NEET Biology Chapter wise Weightage |
Hypotonic Solution FAQs
What are Hypotonic and Hypertonic Solutions?
A hypertonic solution contains a higher concentration of solute than another solution, causing water to move into it. Conversely, a hypotonic solution contains a lower concentration of solute, causing water to move out of it.
What is a Hypertonic Solution?
A hypertonic solution is a type of solution with a higher concentration of solutes outside a cell than inside it.
What is the Difference Between Hypotonic and Isotonic Solutions?
The key difference lies in their osmotic pressures. Isotonic solutions have equal osmotic pressures, while hypotonic solutions have lower osmotic pressures, and hypertonic solutions have higher osmotic pressures.
What is an example of a Hypertonic Solution?
An example is the interior of a red blood cell compared to the solute concentration of fresh water.
Why is Hypertonic Solution Used?
Clinicians use hypertonic fluids to increase intravascular fluid volume and in the treatment of hyponatremia. Hypertonic saline and mannitol are also used to reduce intracranial pressure.
What is a Hypotonic Solution in Class 12?
A hypotonic solution has a lower concentration of solute inside the cell than outside the cell.
What is a hypotonic solution?
A hypotonic solution is one with a lower solute concentration than the cell, causing water to enter and swell the cell. This concept is key in the NEET Biology Syllabus and appears in NEET Biology MCQ and NEET Previous Year Question papers.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.