
Phosphate Formula: Phosphorus, with the atomic number 15, is an element on the periodic table. It comes in two different forms, white phosphorus and red phosphorus, and is commonly found in nature as part of phosphate minerals.
Oxygen, on the other hand, is another element on the periodic table with an atomic number of 8.
Phosphate Formula
Regarding phosphate, its chemical formula is PO 4 3- . It consists of one phosphorus (P) atom and four oxygen (O) atoms. The central phosphorus atom is bonded to the four surrounding oxygen atoms through ionic bonds. This combination is often referred to as a phosphate ion or orthophosphate, and it's a polyatomic ion. In the human body, phosphate is found in bones, teeth, and genetic material.
Phosphate Formula Name
The chemical formula for phosphate is typically written as PO 4 3- . It's also commonly known as the phosphate ion or orthophosphate.Phosphate Formula and Charge
The chemical formula for the phosphate ion is PO 4 3- . It has a charge of -3. This means it has three more electrons than protons, resulting in a negative charge of -3.Phosphate Formula and Valency
The chemical formula for phosphate is PO 4 3- . In this formula, it's important to understand the valency of the elements involved:
Phosphorus (P): Phosphorus, with an atomic number of 15, typically forms compounds where it exhibits a valency of 3 or 5. In the phosphate ion (PO 4 3- ), phosphorus has a valency of 5. This means it can form five chemical bonds with other elements or ions.
Oxygen (O): Oxygen, with an atomic number of 8, usually has a valency of 2. It tends to form two chemical bonds with other elements.
In the phosphate ion (PO 4 3- ), there is one phosphorus atom bonded to four oxygen atoms. The valency of phosphorus (5) and oxygen (2) complements each other to create a stable compound. This combination results in the overall charge of -3 for the phosphate ion, as it has one more negative charge from the three extra electrons than positive charges from protons.
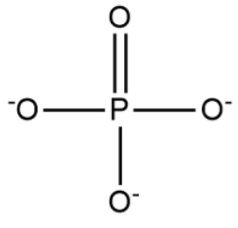
Phosphate Formula Structure
Phosphate has a structure with one phosphorus atom at the center, and around it, there are four oxygen atoms arranged in a tetrahedral shape.
Phosphate Formula Physical Properties
Phosphate has a molecular weight of 94.97 g/mol.
It can accept four hydrogen bonds.
Its conjugate acid form is known as hydrogen phosphate.
Phosphate is somewhat soluble in water.
Chemical Reactions of Phosphate
When phosphate mixes with water, it forms Hydrogen Phosphate (HPO4) and Hydroxide (OH–):
PO 4 3- + H 2 O → HPO 4 2- + OH –
When phosphate reacts with silver, it produces Silver (I) Phosphate:
PO 4 3- + 3Ag → Ag 3 PO 4
When phosphate combines with Nitric acid (HNO 3 ), it results in Nitrate (NO 3 ) and Hydrogen Phosphate (HPO 4 ):
PO 4 3- + HNO 3 → NO 3 + HPO 4 .
Practical Uses of Phosphate
Phosphate helps make your teeth clean and shiny in toothpaste.
It's used in medicines.
Phosphate is important in putting out fires with fire extinguishers.
It helps clean tough stuff in industries.
Tricalcium phosphate is like the magic ingredient in toothpaste that makes it come out of the tube easily and smoothly.
Phosphate, with its chemical formula PO43-, is a crucial chemical compound consisting of one phosphorus atom and four oxygen atoms. It holds a charge of -3 due to its electron configuration. Phosphate plays a significant role in various applications, from dental care and pharmaceuticals to fire extinguishing and industrial cleaning.
Understanding the valency of its constituent elements, phosphorus and oxygen, is essential to grasp its stability and chemical behavior. The phosphate molecule's structure is characterized by a central phosphorus atom surrounded by four oxygen atoms arranged in a tetrahedral shape. Its physical properties, including molecular weight, hydrogen bonding capacity, and solubility, contribute to its versatility.
In chemical reactions, phosphate can engage with other substances to form different compounds, making it versatile in various scientific and industrial processes. Its practical uses extend to creating sparkling fireworks and contributing to a range of essential products and technologies. Overall, phosphate is a fundamental compound with a wide range of applications in both scientific and everyday contexts.
| Related Links | |
| Hyposulfurous Acid Formula | Iodide Formula |
| Molybdic Acid Formula | Iron (II) Oxide Formula |
Phosphate Formula FAQs
What is the chemical formula for phosphate?
What is the charge of the phosphate ion?
What is the valency of phosphorus in the phosphate ion?
Q4. What is the Structure of Phosphate?
What are some physical properties of phosphate?










