Hyposulfurous Acid Formula : Hyposulfurous acid is composed of two hydrogen (H) atoms, one sulfur (S) atom, and two oxygen (O) atoms. Hydrogen (H) holds the atomic number 1 on the periodic table, sulfur (S) is represented by atomic number 16, and oxygen (O) is associated with atomic number 8. Let's discuss the properties of Hyposulfurous acid.
Hyposulfurous Acid Formula
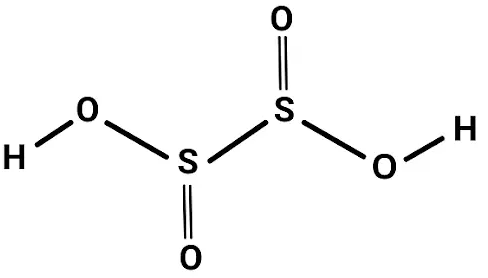
Hyposulfurous acid is characterized by the presence of two hydrogen (H) atoms, one sulfur (S) atom, and two oxygen (O) atoms. This compound serves as an unstable oxoacid of sulfur (S) and occupies an intermediate oxidation state, lying between dithionous acid and hydrogen sulfide. It features two hydroxy groups (OH) that are attached to the sulfur (S) atom. You may also encounter it by various names, including Sulfoxylic acid, Sulfur Dihydroxide, dihydroxidosulfur, or sulfanediol. In the realm of chemical nomenclature, its formula is represented as H 2 SO 2 .
Hyposulfurous Acid Formula Structure
Hyposulfurous acid features two hydroxy groups (OH) bonded to the sulfur (S) atom, which is in a +2 oxidation state.
Preparation of Hyposulfurous Acid
Hyposulfurous acid is encountered in the gaseous phase and serves as an intermediate acid formed through the oxidation of hydrogen sulfide in the natural environment. Additionally, it can be synthesized through several methods, including ultraviolet irradiation of a mixture comprising solid H 2 S and H 2 O, a process occurring in circumstellar disks or comets. Another method involves subjecting solid sulfur dioxide and hydrogen sulfide to ultraviolet irradiation.
Physical Properties of Hyposulfurous Acid
Hyposulfurous acid possesses a molecular weight of 66.07 g/mol and exhibits the presence of the bisulfoxylate ion (SO 2 H – ) along with its conjugate base. It is an isomer of sulfinic acid and boasts a pKa value of 7.97.Chemical Properties of Hyposulfurous Acid
Hyposulfurous acid undergoes disproportionation to yield sulfur (S) and hydrogen sulfite (HSO 3 – ), with the latter further reacting to produce thiosulfate (S 2 O 3 2- ). It can also be oxidized to sulfur dioxide (SO 2 ), forming diothionite (S 2 O 2 4- ).
Hyposulfurous acid exhibits reactivity with dithionite, leading to the formation of hydrogen sulfite (HSO 3 – ) and sulfur (S) in the process:
H 2 SO 2 + S 2 O 4 2- → 2HSO 3 – + S
Moreover, when Hyposulfurous acid interacts with hypochlorite, bromine, or chlorine dioxide, it generates hydrogen sulfite and sulfates as products.
Uses of Hyposulfurous Acid
Hyposulfurous acid is a multifunctional compound used as a reducing agent and bleaching agent in industries ranging from paper to textiles. Its applications span across organic chemistry, food, photography, and polymer manufacturing.
Reducing Agent: It serves as a valuable reducing agent in chemical processes.
Bleaching Agent: In the paper industry, it is employed as a bleaching agent for pulp, contributing to the production of white paper products.
Dyeing: It plays a crucial role in the dyeing process, aiding in the coloration of textiles and other materials.
Organic Chemistry: Hyposulfurous acid is utilized in organic reactions, facilitating the conversion of nitro groups to amino groups.
Industrial Processes: It is integrated into industrial procedures as a component of sulfonating agents and a source of sodium ions.
Textile and Leather Industries: The textile and leather sectors make use of hyposulfurous acid for various purposes.
Food, Photography, and Polymer Industries: It is applied in food processing, photography, and polymer manufacturing processes to achieve specific chemical transformations.
Hyposulfurous acid is a versatile chemical compound with a range of important applications. It acts as a reducing agent in chemical reactions and is used in the paper industry for bleaching pulp. It plays a role in dyeing processes and organic chemistry by converting nitro groups to amino groups. Its industrial applications include sulfonating agents and as a source of sodium ions.
Additionally, it is used in the textile, leather, food, photography, and polymer industries. Its varied uses highlight its significance in numerous sectors of the chemical and manufacturing industries.
| Related Links | |
| Nitrogen Dioxide Formula | Lithium Bromide Formula |
| Lithium Hydroxide Formula | Magnesium Sulfate Formula |
Hyposulfurous Acid Formula FAQs
What is the chemical composition of hyposulfurous acid?
What is the atomic number of sulfur in hyposulfurous acid?
What is the oxidation state of sulfur in hyposulfurous acid?
How is hyposulfurous acid prepared?
What are some other names for hyposulfurous acid?