Methods Of Preparation Of Phenols : Any organic compound that belongs to the phenol family can be identified by its aromatic ring-attached hydroxyl (OH) group to a carbon atom. The most basic member of the family is phenol, also known as carbolic acid or monohydroxy benzene (C 6 H 5 OH).
Additionally, the whole family is referred to by the generic term, phenol. Phenols and alcohols are comparable in that they form stronger hydrogen bonds. They are therefore more soluble in water and have higher boiling points than alcohols. An overview of the synthesis of phenols from haloarenes, cumene, diazonium salts, and benzene sulphonic acid is given in this article. An organic compound known as phenol has a hydroxyl group and a benzene ring attached to it. Being weak acids, phenols typically lose one positive hydrogen ion (H + ) from the hydroxyl group to form phenoxide ions. Historically, coal tar was the main ingredient used to make phenol. But as technology has advanced, new techniques for preparing phenols have been developed. The main source of phenol produced in labs is benzene derivatives. Below is an explanation of a few of the phenol preparation techniques.
Preparation of Phenols
Preparation of Phenol s: Nowadays, phenol is synthesized and sold commercially. In the laboratory, one of the following techniques is used to create phenols from benzene derivatives.
Preparation of phenols form Haloarenes
Preparation of phenols form Haloarenes: - The mono substitution of the benzene ring results in the formation of haloarenes, such as chlorobenzene. At 623 K and 300 atm, sodium hydroxide and chlorobenzene fuse to form sodium phenoxide. Finally, acidification produces phenol form sodium, phenoxide.
Elevated temperatures can be used to treat this benzene sulphonic acid in order to promote the formation of sodium phenoxide by melting sodium hydroxide. Finally, acidification produces phenol from sodium phenoxide.
Preparation of phenols form Benzene Sulphonic Acid
: - A reaction between benzene and oleum yields benzene sulphonic acid. Elevated temperatures can be used to treat this benzene sulphonic acid in order to promote the formation of sodium phenoxide by melting sodium hydroxide. Finally, acidification produces phenol from sodium phenoxide.
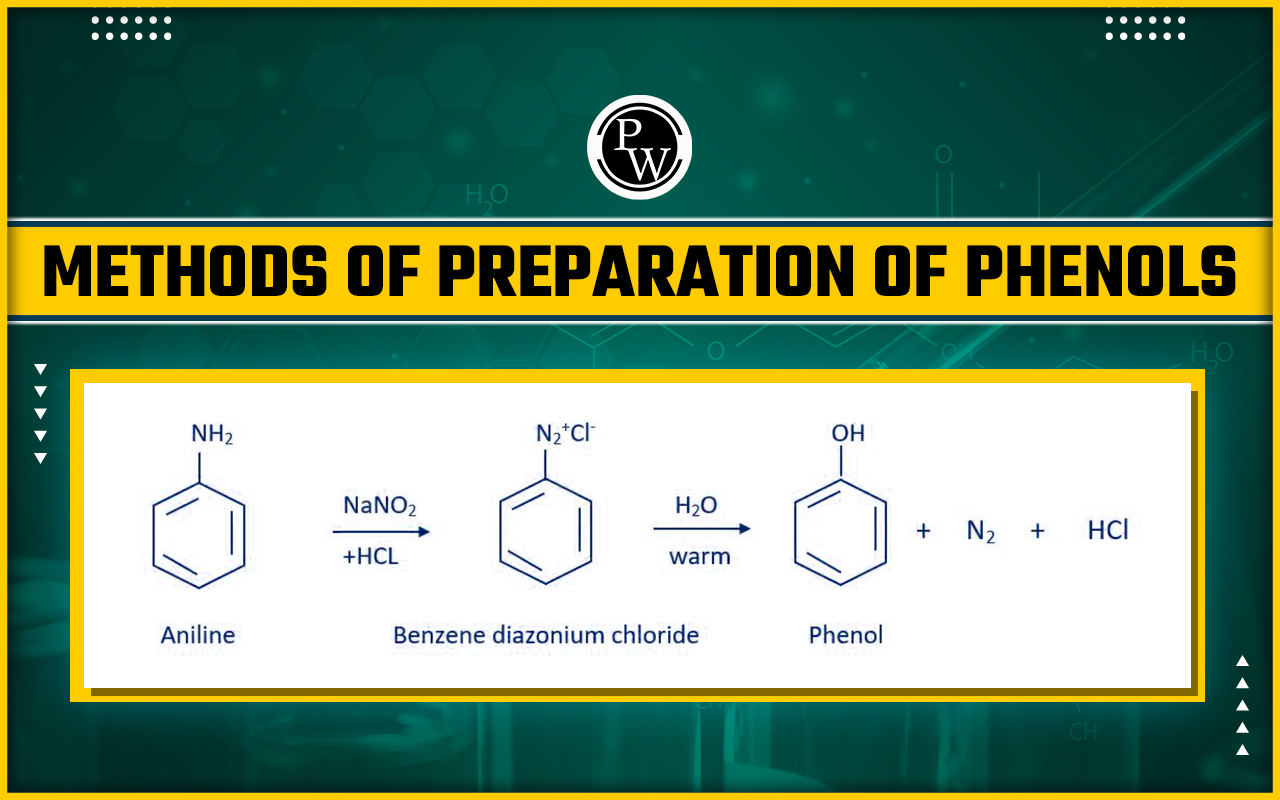
Preparation of phenols form Diazonium salts
Preparation of phenols form Diazonium salts: - Diazonium salts are produced by treating an aromatic primary amine at 273 –278 K with nitrous (NaNO 2 + HCl) acid. These salts of diazonium have a very reactive nature. Finally, these diazonium salts hydrolyze phenols when heated with water. By treating diazonium salts with diluted acids, phenols can also be extracted from them.
Preparation of phenols form Cumene
Preparation of phenols form Cumene: - Friedel-Crafts alkylation of benzene with propylene yields cumene, an organic compound. When cumene (isopropyl benzene) is exposed to air, it oxidizes and produces cumene hydroperoxide. Phenols are produced when diluted acid is used to further treat cumene hydroperoxide. As a significant byproduct of this reaction, acetone is also produced in significant amounts. Therefore, purifications are necessary for phenols prepared using these methods.
Methods Of Preparation Of Phenols FAQs
Q.1: Why are phenols less soluble in water?
Q. 2: What are the methods of preparation of Phenols?
Q.3: What is the use of Phenols?
Q.4 : How to prepare Phenols from Cumenes?
Q.5 : Why is phenol acidic?