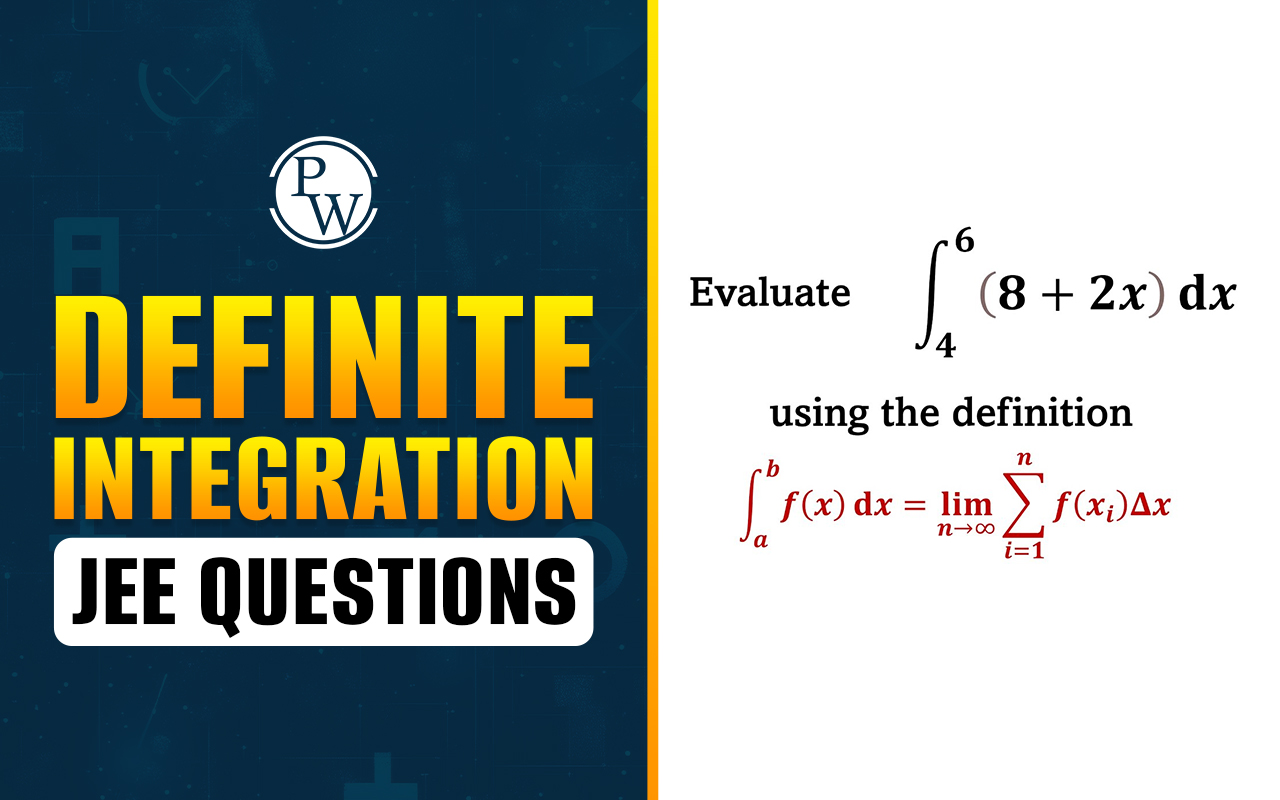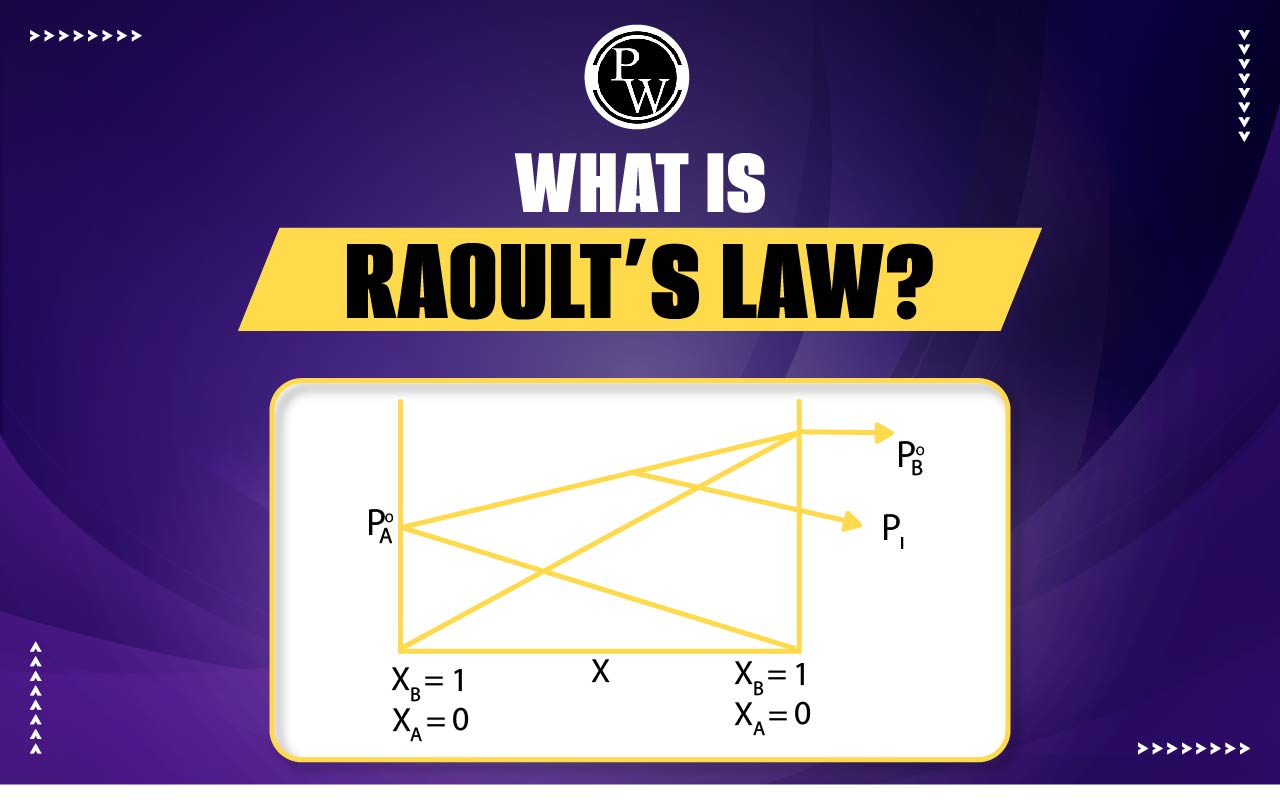
What Is Raoult’s Law? : Raoult’s Law, which discusses the relationship between ideal liquid mixture composition and vapour pressure, is an important concept in chemistry. The French scientist François-Marie Raoult first presented it in 1887. This law discusses the relationship between a part's pressure and its percentage in the mixture as a whole.
To put it simply, Raoult's Law explains the relationship between a substance's pressure and its total concentration in a mixture. Some liquid combinations follow this rule throughout an extensive concentration range, whereas this law is applicable exclusively to diluted solutions. Achieve JEE excellence with PW JEE Online Course . Join now to fulfill your engineering dreams!What is Raoult’s Law?
What is Raoult’s Law? : According to Raoult's law, the partial vapour pressure of a solvent in a mixture or solution is the same as or equal to the vapour pressure of the pure solvent multiplied by the solvent's mole fraction in the mixture. Raoult's law equation is expressed mathematically as: Psolution = XsolventP0solvent Where, Psolution = the solution's vapour pressure Χsolvent = solvent's mole fraction P0solvent is the pure solvent's vapour pressure. Also, by going over the example below, we will understand the underlying concept of the law. Think of a container containing a solution of the volatile liquids A and B. There would be particles of both A and B in the vapour phase as they are both volatile. As a result, the partial pressure that the vapour particles of A and B exert helps to raise the overall pressure above the solution.Some Basic Terms to Learn
- Solution: A solution is a homogenous mixture that contains two or more components.
- Solute: The substance that has been dissolved in the solvent is referred to as the solute.
- Solvents: A Solvent is a substance that dissolves the solute.
- Mole Fraction: The mole fraction is the ratio of a component's moles to the total moles in the solution.
- Vapour Pressure: Vapour pressure is the pressure exerted by a substance's vapour when in equilibrium with its liquid or solid form.
- Partial vapour pressure: When multiple liquids are combined, each adds to the total pressure exerted by the vapour above the combination. This contribution from each liquid is known as the partial vapour pressure.
- Mole fraction : The number of moles of a component divided by the total number of moles in the mixture. It is a dimensionless value between 0 and 1.
Importance of Raoult’s law
Importance of Raoult’s law : Let's suppose we have a closed container that contains liquid A, which is volatile. Vapour particles of A will begin to develop after a while as a result of evaporation. Then, as time goes on, the liquid particles (on the surface) and the vapour particles of A will be in dynamic equilibrium. The vapour pressure of A at a given temperature is the force that its vapour particles exert at that temperature.- All solids and liquids display vapour pressure, which is solely dependent on the kind of liquid and temperature.
- Suppose for a moment that we are filling this container with liquid B (solute). As a consequence, on the solution's surface, B particles will take up the space between A particles.
- A portion of the molecules on the surface of any given liquid will have enough energy to escape into the vapour phase.
- Now that there are fewer A particles on the surface, there will be less A vapor particles in the vapour phase. As a consequence, A's vapour pressure will decrease.
- In comparison to pure liquid B, there will be less B particles in the vapour phase if we now assume that B is also volatile.
- Raoult's law determines this new pressure, or partial pressure, of each (A and B), which is dependent upon the concentration of each component in the liquid phase.
Looking For Best Books for the JEE Exam Preparation?
Relationship of Raoult’s Law With Other Laws
This law is similar to other laws in the following ways:Similarity to Ideal Gas Law:
- Raoult's law is quite similar to the ideal gas law.
- But, it specifically applies to solutions, not gases.
Exception to Ideal Gas Law:
- Ideal gas law assumes no intermolecular forces between dissimilar molecules.
- Raoult’s law assumes equal intermolecular forces between different and similar molecules in solutions.
Applicability to Non-Ideal Solutions:
- Raoult’s law is versatile and can be used for non-ideal solutions.
- In such cases, various factors need consideration, especially interactions between molecules of different substances.
Ideal System Derivation:
- A perfectly ideal system involves an ideal liquid and ideal vapour.
- Combining Raoult’s law and Dalton’s law in this scenario yields a valuable equation.
Limitation of Raoult’s Law
There are some limitations to Raoult's law.- Raoult’s law is useful for describing ideal solutions. However, perfect solutions are hard to find, and they are rare. Different chemical components have to be chemically similar equally.
- Since many of the liquids that are in the mixture do not have the same consistency in terms of pulling forces, these types of solutions tend to stray away from the law.
Application of Raoult’s Law
Estimating a solution's boiling and freezing points: By calculating the mole fraction of a solute, we may determine how much a solution's boiling and freezing points have changed from those of the pure solvent. In many industrial operations and scientific research, this is essential. Determination of concentration: We may indirectly determine the solute's concentration by monitoring the solution's decrease in vapour pressure. Pharmaceutical and analytical chemistry applications employ this method. Raoult's law is essential to understanding osmotic pressure, which is a crucial component of many separation procedures, including dialysis and reverse osmosis.Raoult’s Law FAQs
Q.1 : Give a particular application of Raoult’s law.
Ans. To measure the bonding strength of liquids.
Q.2 : What are the 3 laws of chemistry?
Ans. Three laws in chemistry are considered as the "fundamental chemistry laws," and they are as follows: The law of conservation of mass. The law of constant proportions. The law of multiple proportions.
Q.3 : What is the basic law of chemistry?
Ans. Many people refer to the law of conservation of mass as the most crucial concept in chemistry.
Q.4 : What is the pressure law?
Ans. According to pressure law, the absolute temperature of a certain amount of gas at constant volume determines its pressure.
Q.5 : Who named chemistry?
Ans. It is thought that the term "chemistry" originated in either ancient Greece or Egypt.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.